Core Level Spectroscopies in Charge Transfer Multiplet Program
Core level spectroscopies in the Charge Transfer Multiplet Program are essential for analyzing d and f-shells in various spectroscopic techniques involving excitation, decay, resonances, and scattering. These spectroscopies include X-ray spectroscopies, electron spectroscopies, and resonant spectroscopies, each providing unique insights into the electronic structure and interactions of materials at the atomic level.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
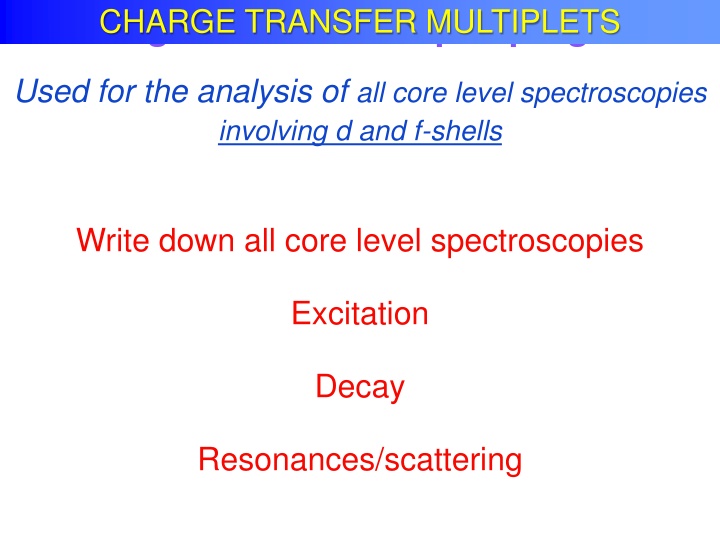
Charge Transfer Multiplet program CHARGE TRANSFER MULTIPLETS Used for the analysis of all core level spectroscopies involving d and f-shells Write down all core level spectroscopies Excitation Decay Resonances/scattering
X-ray spectroscopies 3d8 + XPS XAS 2p53d8+e 2p53d9
X-ray spectroscopies 3d8 + XPS XAS 2p53d8+e 2p53d9 XES 3d8 + RIXS
X-ray spectroscopies 3d8 + XPS XAS 2p53d8+e 2p53d9 XES AES 3p43d9+e 3d8 + RIXS RAES
X-ray spectroscopies 3d8 + XPS XAS 2p53d8+e 2p53d9 XES AES 3p43d9+e 3d8 + 3d7 + e RIXS RAES RPES
X-ray spectroscopies 3d8 + XPS XAS 2p53d8+e 2p53d9 XES XES AES 3p43d9+e 3d8 + 3d7 +e + 3d7 + e RIXS RAES RPES XES
X-ray spectroscopies 3d8 + XPS2 XAS1 2p53d8+e 2p53d9 XES3 XES AES4 IXS 8 3p43d9+e 3d8 + 3d7 +e + 3d7 + e RIXS5 XES RAES6 RPES7
Electron spectroscopies 3d8 + e APS 2p53d10
Electron spectroscopies 3d8 + e APS 2p53d10 XES 3d9+ RIPES
Electron spectroscopies 3d8 + e APS 2p53d10 XES AES 3d8+e 3d9+ RIPES REELS
Electron spectroscopies 3d8 + e 1. RIXS 2. RAES 3. RPES 4. XES 5. RIPES 6. REELS APS9 EELS12 2p53d10 XES AES 3d8+e 3d9+ RIPES10 REELS11
Resonant spectroscopies 1. RIXS ( , ) 2. RAES 3. RPES 4. XES 5. RIPES 6. REELS (+ coincidences) (+ polarizations) (+ momentum) (+ time, space) RIXS X-ray out ( ) X-ray in ( )
Resonant spectroscopies 1. RIXS ( , ) 2. RAES ( ,e ) 3. RPES 4. XES 5. RIPES 6. REELS (+ coincidences) (+ polarizations) (+ momentum) (+ time, space) RAES Electron out (e ) X-ray in ( )
Resonant spectroscopies 1. RIXS ( , ) 2. RAES ( ,e ) 3. RPES ( ,e ) 4. XES ( ,e ) 5. RIPES 6. REELS (+ coincidences) (+ polarizations) (+ momentum) (+ time, space) XES Electron out (e ) X-ray out ( )
Resonant spectroscopies 1. RIXS ( , ) 2. RAES ( ,e ) 3. RPES ( ,e ) 4. XES ( ,e ) 5. RIPES (e, ) 6. REELS (e,e ) (+ coincidences) (+ polarizations) (+ momentum) (+ time, space) XES Electron out (e ) X-ray out ( )
Charge Transfer Multiplet program CHARGE TRANSFER MULTIPLETS Used for the analysis of XAS, EELS, Photoemission, Auger, XES involving d and f-shells ATOMIC PHYSICS GROUP THEORY MODEL HAMILTONIANS
3d XAS of rare earths 4f electrons are localized No effect of surroundings (crystal field < lifetime broadening) 3d XAS is self screened > no charge transfer effect Initial state electron-electron interaction. Valence spin-orbit coupling Final state + core hole valence hole multiplet interaction. + core hole spin-orbit coupling
3d XAS of rare earths Nd3+ 4f3 system: ground state is 4I9/2
2p XAS of 3d transition metal oxides 3d electrons are less localized Effect of surroundings (crystal field effect) 3d XAS is self screened > weak charge transfer effect Initial state electron-electron interaction. valence spin-orbit coupling crystal field effect Final state core hole valence hole multiplet interaction. core hole spin-orbit coupling crystal field effect
Crystal Field Effects crystal field effect eg states t2g states
Comparison with Experiment 2p XAS of ScF3 with crystal field multiplets PHYS. Rev. B. 41, 928 (1990)
Atomic Multiplet Theory X-ray absorption from 3d0 to 2p53d1 1P 1P1 Dipole selection rule: J=-1, 0 or +1 J=J 0 1D 1D2 1F 1F3 3P2 3P 3P1 3d0 1S 1S0 3P0 2p53d1 3D3 3D 3D2 3D1 3F4 3F3 3F 3F2
Octahedral crystal field splitting CRYSTAL FIELD EFFECT 2T2g 2Eg 3d1 2D metal ion in free space in symmetrical field in octahedral ligand field eg x2-y2 z2 t2g yz xz xy x2-y2 yz z2 xz xy
Atomic Multiplet Theory CRYSTAL FIELD EFFECT J=0 J=1 A1 T1 T2 E A2 T1 T2 A1 T1 T2 E Branching rules: J=2 Same rules for any quantum number S, L or J 3dN J=3 J=4
Atomic Multiplet Theory X-ray absorption from 3d0 to 2p53d1 1P 1P1 T1 1D 1D2 T2 E 1F 1F3 A2 T1 T2 3P2 T2 E 3P 3P1 T1 ee LS CFS 2p53d1 3P0 3d0 1S 1S0 A1 A1 3D3 A2 T1 T2 3D 3D2 T2 E 3D1 T1 3F4 A1 T1 T2 E 3F 3F3 A2 T1 T2 3F2 T2 E
Atomic Multiplet Theory X-ray absorption from 3d0 to 2p53d1 1P 1P1 T1 1D 1D2 T2 E 1F 1F3 A2 T1 T2 3P2 T2 E 3P 3P1 T1 ee LS CFS 2p53d1 3P0 3d0 1S 1S0 A1 A1 A2 T1 T2 3D3 3D 3D2 T2 E T1 3D1 3F4 A1 E T1 T2 3F 3F3 A2 T1 T2 3F2 T2 E 2 3 7 8 5
Atomic Multiplet Theory X-ray absorption from 3d0 to 2p53d1 Dipole selection rule: J=T1 (cubic symmetry) 1P 1P1 T1 1D 1D2 T2 E 1F 1F3 A2 T1 T2 3P2 T2 E 3P 3P1 T1 ee LS CFS 2p53d1 3P0 3d0 1S 1S0 A1 A1 A2 T1 T2 3D3 3D 3D2 T2 E T1 3D1 3F4 A1 E T1 T2 3F 3F3 A2 T1 T2 3F2 T2 E 2 3 7 8 5
Comparison with Experiment 2p XAS of ScF3 with crystal field multiplets PHYS. Rev. B. 41, 928 (1990)
Multiplet calculations ATOMIC valence e-e interactions Fdd core-valence e-e FpdGpd core & valence spin-orbit 4f, 5f SYMMETRY crystal field 10Dq, Ds, Dt molecular field, M or H e-e screening ionic 3d (4d, 5d) BONDING charge transfer , U, Q hopping T covalent 3d mixed valence f
Multiplet calculations ATOMIC valence e-e interactions Fdd core-valence e-e FpdGpd core & valence spin-orbit 4f, 5f 3d ions SYMMETRY crystal field 10Dq, Ds, Dt molecular field, M or H e-e screening ionic 3d (4d, 5d) BONDING charge transfer , U, Q hopping T covalent 3d mixed valence f
Charge Transfer Effects Charge transfer effects Hubbard U for a 3d8 ground state: U= E(3d7) + E(3d9) E(3d8) E(3d8) Ligand-to-Metal Charge Transfer (LMCT): = E(3d9L) E(3d8)
Charge Transfer Effects Charge transfer effects Main screening mechanism in XAS of oxides: Ligand-to-metal charge transfer Charge transfer energy is important for XAS Hubbard U is NOT important for XAS spectral shape (It is important for (e.g.) XPS spectral shapes)
Charge transfer effects in XAS and XPS transition metal oxides Ground state: 3d8 + 3d9L Energy of 3d9L: Charge transfer energy 3d9L 3d8 Ground State
Charge Transfer Effects 3d9L 3d8
Charge Transfer Effects 9 3d9L 8 3d8
Charge Transfer Effects 3d8 - 3d9L 3d9L 3d8 3d8 + 3d9L
Charge Transfer Effects Charge transfer effects NiO: Ground state: 3d8 + 3d9L Energy of 3d9L: Charge transfer energy 3d9L 2p53d10L +U-Q 2p53d9 3d8
Charge transfer effects 3d8 - 3d9L c3d9 c3d10L ~0 +U-Q ~1 3d8 + 3d9L ' c3d9 + c3d10L Intensity bonding combination: [ + ]2 ( 2+ 2)2=1
Charge transfer effects 3d5 - 3d6L c3d6 c3d7L ~0 +U-Q ~1 3d5 + 3d6L ' c3d6 + c3d7L Intensity anti-bonding combination: [ - ]2 ( )2=0
Charge Transfer Effects Charge transfer effects Neutral experiments are self-screened XAS, optical, EPR, EELS, RIXS >> small screening satellites >> crystal field theory can be used Ionising experiments are not self-screened XPS, Auger, >> large screening > large satellites >> crystal field theory can not be used
Charge transfer effects in XAS and XPS Charge transfer effects in XAS and XPS Transition metal oxide: Ground state: 3d5 + 3d6L Energy of 3d6L: Charge transfer energy 3d6L XPS XAS 2p53d5 3d5 2p53d7L -Q Ground State +U-Q 2p53d6L 2p53d6
Charge Transfer Effects Charge transfer effects NiO: Ground state: 3d8 + 3d9L Energy of 3d9L: Charge transfer energy 3d9L 2p53d10L +U-Q 2p53d9 3d8
Tanabe-Sugano with Charge transfer Tanabe-Sugano diagrams with charge transfer 3d8 + 3d9L NiO Cu3+
Charge Transfer effects Charge transfer effects in XAS 3d8 + 3d9L =10 30% 3d8 1A1 =5 NiO =0 30% 3d8 La2Li Cu O4 =-5 3A2 =-10 Chem. Phys. Lett. 297, 321 (1998)
LMCT and MLCT: - bonding FeIII: Ground state: 3d5 + 3d6L N N N N C C C C M M M M C C C C M M M M N N N N filled - orbital orbital orbital orbital orbital filled - filled - filled - filled empty d or p empty d or p empty d or p empty empty d or p orbital orbital orbital orbital d-orbital filled d-orbital d-orbital d-orbital d-orbital filled filled filled empty *-orbital *-orbital *-orbital *-orbital empty empty empty 3d6L (i) - (i) (i) - (i) 2p53d7L (ii) back-donation (i) donation +U-Q C ? C?N distance C ? C ? C?N distance C ? 2p53d6 3d5 with Ed Solomon (Stanford) JACS 125, 12894 (2003), JACS 128, 10442 (2006), JACS 129, 113 (2007)
LMCT and MLCT: - bonding FeIII: Ground state: 3d5 + 3d6L + 3d4L 2p53d5L 3d4L -U+Q + 2 3d6L 2p53d7L +U-Q - 2 2p53d6 3d5 with Ed Solomon (Stanford) JACS 125, 12894 (2003), JACS 128, 10442 (2006), JACS 129, 113 (2007)
LMCT and MLCT: - bonding FeIII(tacn)2 10 Fit X Series2 Normalized Absorption 8 FeIII(CN)6 6 4 2 0 700 705 710 715 720 725 730 -2 with Ed Solomon (Stanford) JACS 125, 12894 (2003), JACS 128, 10442 (2006), JACS 129, 113 (2007) Energy (eV)
Multiplet calculations Calculated for an atom/ion Valence and core spin-orbit coupling Core and valence electron-electron interaction. Comparison with experiment Core hole potential and lifetime Local symmetry (crystal field) Spin-spin interactions (molecular field) Core hole screening effects (charge transfer)
First Principle Multiplet calculations Calculated for an atom/ion Valence and core spin-orbit coupling Core and valence electron-electron interaction. Comparison with experiment Core hole potential and lifetime Local symmetry (crystal field) Spin-spin interactions (molecular field) Core hole screening effects (charge transfer)
2p XAS first-principle codes SOLIDS Band structure multiplet (Haverkort, Green, Hariki) Cluster DFT multiplet (Ikeno, Ramanantoanina, Delley) MOLECULES Restricted Active Space CI (Odelius, Kuhn) Restricted Open-shell CI (Neese) TDDFT/BSE Time-Dependent DFT (Joly) Bethe-Salpeter (Rehr, Shirley) Multi-channel Multiple-scattering (Kruger)