Comparison of INSTI vs PI
Study WAVES compares the efficacy of EVG/C/FTC/TDF versus ATV/r/FTC/TDF in women with ARV-naïve HIV. The study assesses virologic success, CD4 cell count changes, and response rates at week 48. Baseline characteristics and patient disposition, as well as HIV RNA levels, CD4 cell counts, and treatment responses, are analyzed.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Comparison of INSTI vs PI FLAMINGO GS-236-0103 ACTG A5257 WAVES ARIA
Study WAVES: EVG/C/FTC/TDF QD vs ATV + r + FTC/TDF QD in Women Design Randomisation* 1 : 1 Double-blind W48 EVG/C/FTC/TDF 150/150/200/300 mg QD Women ARV-na ve HIV RNA > 500 c/mL Any CD4 cell count Sensitivity to FTC, TDF and ATV eGFR > 70 mL/min N = 289 Open-label extension ATV + r + TDF/FTC placebo ATV + r 300/100 mg + FTC/TDF QD N = 286 EVG/C/FTC/TDF placebo *Randomisation was stratified by HIV RNA (< 100,000 or 100,000-400,000 or > 400,000 c/mL) at screeningand race (black or non-black) Objective Non inferiority of EVG/C/FTC/TDF at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (lower margin of the 2-sided 95% CI for the difference = -12%) Squires K. Lancet HIV 2016; 3(9):e410-e420 WAVES
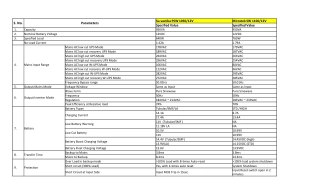
Study WAVES: EVG/C/FTC/TDF QD vs ATV + r + FTC/TDF QD in Women Baseline characteristics and patient disposition EVG/C/FTC/TDF N = 289 ATV + r + FTC/TDF N = 286 Female Median age, years White / Black / Asian AIDS HIV RNA (log10 c/mL), median (Q1-Q3) HIV RNA 100,000-400,000 c/mL HIV RNA 400,000 c/mL CD4 cell count (/mm3), median CD4 < 200 per mm3 Hepatitis B / hepatitis C coinfection Discontinuation by W48, N (%) For lack of efficacy For adverse event Lost to follow-up / Withdrew consent Non-compliance / Other 100% 34 100% 35 44% / 50% / 3% 4% 4.46 (4.09-4.97) 42% / 47% / 6% 5% 4.56 (4.02-5.00) 18% 8% 370 18% 3% / 9% 45 (16%) N = 1 N = 19 N = 12 / N = 5 N = 5 / N = 3 15% 9% 344 17% 4% / 8% 29 (10%) N = 0 N = 5 N = 12 / N = 6 N = 4 / N = 2 Squires K. Lancet HIV 2016; 3(9):e410-e420 WAVES
Study WAVES: EVG/C/FTC/TDF QD vs ATV + r + FTC/TDF QD in Women Response to treatment at week 48 HIV RNA < 50 c/mL (ITT, snapshot) % 100 87 81 EVG/C/FTC/TDF (N = 289) ATV + r + FTC/TDF (N = 286) 75 50 25 12 9 7 4 0 Virologic success No virologic data Virologic failure Adjusted difference (95% CI) = 6.5 % (0.4 ; 12.6) p = 0.034 Mean CD4/mm3increase at W48 : + 221 (EVG/C/FTC/TDF) vs + 212 (ATV + r + FTC/TDF) Squires K. Lancet HIV 2016; 3(9):e410-e420 WAVES
Study WAVES: EVG/C/FTC/TDF QD vs ATV + r + FTC/TDF QD in Women HIV RNA < 50 c/mL at W48 by baseline HIV RNA and CD4 count EVG/C/FTC/TDF ATV + r + FTC/TDF % 100 90 88 87 86 86 82 82 81 79 78 75 50 25 286 286 220 214 69 146 131 143 154 72 0 Overall < 100,000 > 100,000 < 350 > 350 HIV RNA (copies/mL) CD4 cell count (/mm3) Squires K. IAS 2015 Vancouver, Abs. MOLBPE08 ; Squires K. Lancet HIV 2016; 3(9):e410-e420 WAVES
Study WAVES: EVG/C/FTC/TDF QD vs ATV + r + FTC/TDF QD in Women Emergence of resistance EVG/C/FTC/TDF ATV + r + FTC/TDF Resistance analysis population 19 21 Final RAP* 7 12 Resistance mutations emergence 0 3 NRTI-resistance 1 3 D67D/N 1 0 M184V/I 0 3 K65R 0 0 INSTI-resistance 0 0 Primary PI-resistance 0 0 * Criteria : - Suboptimal response (HIV RNA 50 c/mL and < 1 log10reduction from baseline by W8, confirmed) - Virologic rebound (> 400 c/mL after achieving HIV RNA < 50 c/mL, or 2 consecutive visits with > 1 log10increase from nadir) - HIV RNA > 400 c/mL at W48 Exclusion of patients with HIV RNA < 50 c/mL at subsequent visits Squires K. Lancet HIV 2016; 3(9):e410-e420 WAVES
Study WAVES: EVG/C/FTC/TDF QD vs ATV + r + FTC/TDF QD in Women Renal and bone mineral density (DXA) assessments EVG/C/FTC/TDF ATV + r + FTC/TDF p DXA assessment at baseline, spine ; hip, N at W48, spine ; hip, N 138 ; 120 136 ; 118 150 ;128 150 ; 128 Median change from baseline in BMD Lumbar spine - 3.23 % - 3.28 % 0.69 Hip - 2.99 % - 2.68 % 0.37 Median change from baseline in eGFR (mL/min), Cockroft-Gault formula - 6.1 - 2.4 0.15 Median change from baseline in fasting lipids (mg/dL) EVG/C/FTC/TDF ATV + r + FTC/TDF p Total cholesterol + 7 + 2 0.02 Other lipid parameters (LDL-c, HDL-c, Triglycerides, Total cholesterol:HDL-cholesterol ratio ns Squires K. Lancet HIV 2016; 3(9):e410-e420 WAVES
Study WAVES: EVG/C/FTC/TDF QD vs ATV + r + FTC/TDF QD in Women Adverse events and Grade 3-4 laboratory abnormalities EVG/C/FTC/TDF 5 discontinuations 1 3 1 0 2 0 ATV + r + FTC/TDF 19 discontinuations 4 4 0 2 9 1 0 15 15 14 14 11 / 12 Adverse events leading to discontinuation, N Hepatobiliary disorder Gastro-intestinal disorder Pulmonary tuberculosis Renal Skin disorder Drug hypersensitivity Adverse event in 10% of patients, % Headache Upper respiratory tract infection Nausea Vomiting Jaundice / Icterus Grade 3-4 laboratory abnormalities in 2%, % Serum amylase elevation Neutropenia < 1000/mm3 ALT elevation Hyperbilirubinemia Glycosuria 16 16 15 10 < 1 / < 1 2 2 2 2 3 2 46 2 < 1 0 Squires K. Lancet HIV 2016; 3(9):e410-e420 WAVES
Study WAVES: EVG/C/FTC/TDF QD vs ATV + r + FTC/TDF QD in Women Summary EVG/C/FTC/TDF QD was virologically non inferior and superior to ATV + r + FTC/TDF Similar virologic response of the 2 regimens in different subgroups of patients, including those with high HIV RNA or CD4 < 350/mm3 at enrolment Development of major resistance mutations occurred in No patients on EVG/C/FTC/TDF 3 patients on ATV + r + /FTC/TDF: NRTI mutations, no PI mutations Discontinuation because of adverse events was lower with EVG/C/FTC/TDF Less incidence of icterus and hyperbilirubinemia with EVG/C/FTC/TDF Comparable changes in fasting lipids in both groups, except for total cholesterol which elevation was higher with EVG/C/FTC/TDF Median decreases in estimated glomerular filtration rate and in spine and hip BMD were modest and not different between the 2 groups Squires K. Lancet HIV 2016; 3(9):e410-e420 WAVES