Comparative Analysis of Amide, Imide, and Urea Compounds in Chemical Testing
This analysis delves into the properties and chemical reactions of amide, imide, urea, and related compounds like sulphanilamide and phthalimide. It explores their characteristics such as odor, solubility, and reactions to different tests like acid-base testing and preliminary examinations. The discussion also includes specific tests like the biuret reaction for urea to distinguish these compounds based on their unique features.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
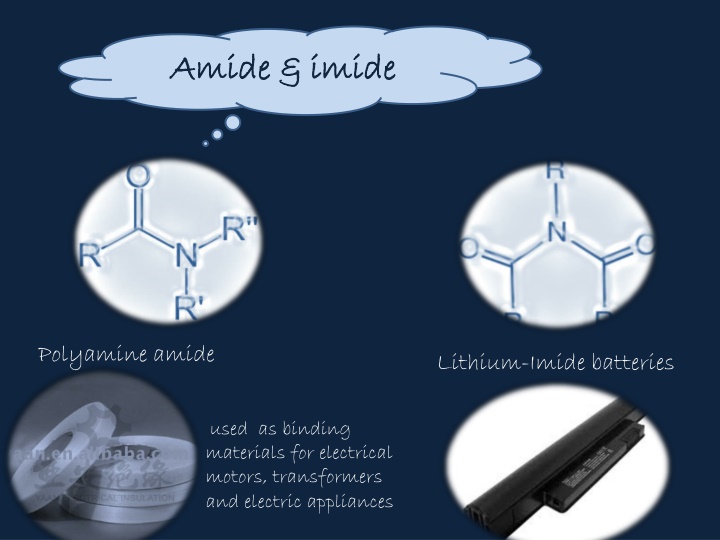
Amide & Amide & imide imide Polyamine amide Lithium-Imide batteries used as binding materials for electrical motors, transformers and electric appliances
sulphanilamide sulphanilamide phthalimide phthalimide urea urea structure structure State and State and color color Solid Solid white white Solid fine powder Solid fine powder white white Solid fine powder Solid fine powder white white Granules Granules odor odor odorless odorless Dry heat test Dry heat test Inflammable Inflammable Non Non luminos luminos No No smoky smoky Solidification Solidification Inflammable Inflammable Luminous Luminous smoky smoky Inflammable Inflammable Luminous Luminous smoky smoky Apperance Apperance change change Odor change Odor change melting melting Ammonia odor Ammonia odor Flame color Flame color No change No change Violet fumes Violet fumes residue residue White residue White residue No residue No residue
Acid base characte Acid base character H H2 2O O L.P L.P HCL HCL NaOH NaOH Na Na2 2CO CO3 3 No Urea soluble - - - neutral change Not done Not done sulphanilamide Insoluble soluble soluble - ? Soluble under heat (cution) Weak phthalimide insoluble insoluble acidic effervescent
sulphanilamide sulphanilamide Base test: Base test: .+dil dil HcL HcL soluble 30% %NaOH NaOH NOT PPT. Acid test: Acid test: 10 % %NaOH NaOH soluble + conc. conc. Hcl Hcl NOT ppt Sulph Sulph.+ 30 soluble + NOT PPT. + Sulph.+ Sulph.+10 + soluble NOT ppt. Amphoteric Amphoteric Not true acid Not true acid Not true base Not true base
Preliminary test Preliminary test urea urea sulphanilamide sulphanilamide No change Ammonia odor No change Ammonia odor No reaction phthilimide phthilimide Soda lime On cold On hot On cold On hot On cold & on hot On cold &on hot 30% NaOH fecl3 H2SO4 No reaction
General and specific General and specific test test urea urea Biuret reaction: Biuret reaction: In dry test tube put few granules of urea then heat on flame until melt and solidify to give white residue. After that cool test tube + 10% NaOH drop by drop to dissolve it and give clear solution then add+ cupper sulphate purple color
Urea oxalate formation: Urea oxalate formation: Saturated solution of urea + 1ml oxalic acid urea oxalate as white crystalline ppt. (shiny). Urea nitrate formation: Urea nitrate formation: Saturated solution of urea + Conc. Nitric acid urea nitrate as white ppt.
sulphanilamide sulphanilamide Amphoteric property Amphoteric property Acid and base test. Acid and base test. Azo Azo dye test: dye test: 1 1- -Sulph. + Sulph. + dil ice ice dil Hcl Hcl to dissolve it +NaNO to dissolve it +NaNO2 2 Put in Put in 2 2- -phenol + phenol +10 10 % % NaOH NaOH put in ice put in ice After After 5 5 minutes pour minutes pour 1 1 on on 2 2 red orange dye red orange dye. .
phthalimide phthalimide Phthaline Phthaline test: test: Phth. + phenol + Conc. H2SO4 heat until fusions after cooling pour it in beaker contain 10 ml of 10% NaOH unstable pink color. Fluorescence test Fluorescence test: Phth. + resercinol +Conc. H2SO4 heat until fusion then cooling and pour it in beaker contain 10 ml 10% NaOH reddish brown color give green fluorescence
IR location IR location Amid stretching band at 1690 cm-1