Cenicriviroc in NASH Phase 2b Study
Phase 2b study evaluating cenicriviroc in NASH patients, focusing on hepatic histologic improvement, fibrosis, and metabolic factors. The study design, randomization, baseline characteristics, primary and key secondary endpoints, and efficacy outcomes are discussed.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
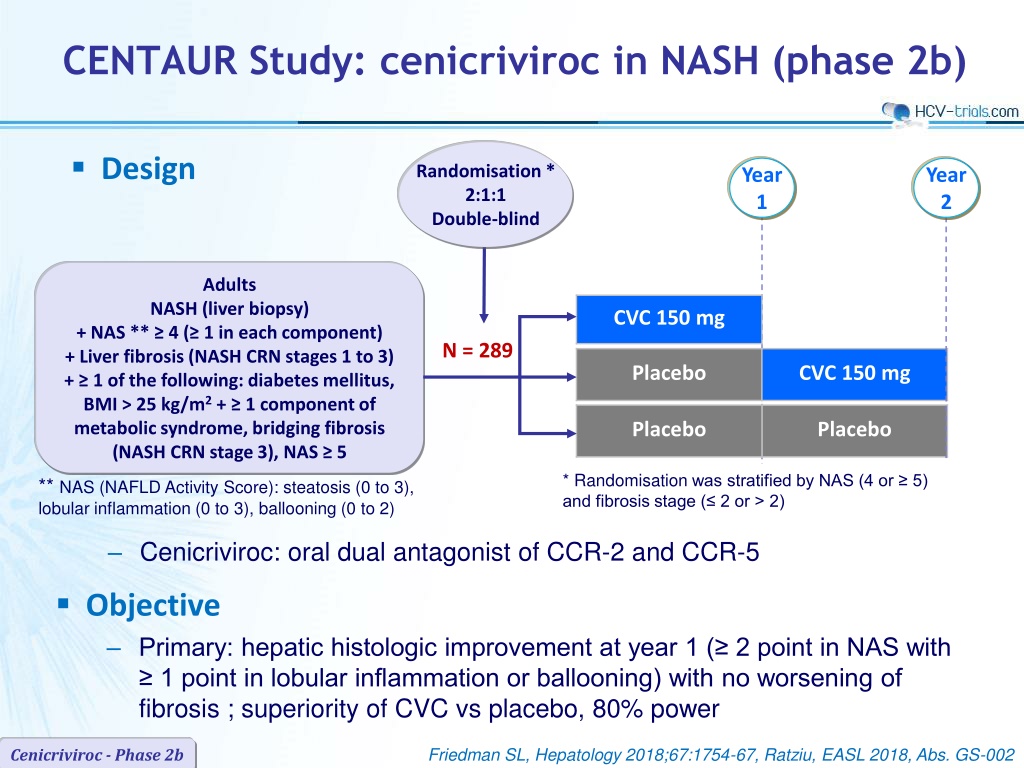
CENTAUR Study: cenicriviroc in NASH (phase 2b) Design Randomisation* 2:1:1 Double-blind Year 1 Year 2 Adults NASH (liver biopsy) + NAS ** 4 ( 1 in each component) + Liver fibrosis (NASH CRN stages 1 to 3) + 1 of the following: diabetes mellitus, BMI > 25 kg/m2+ 1 component of metabolic syndrome, bridging fibrosis (NASH CRN stage 3), NAS 5 CVC 150 mg N = 289 Placebo CVC 150 mg Placebo Placebo * Randomisation was stratified by NAS (4 or 5) and fibrosis stage ( 2 or > 2) ** NAS (NAFLD Activity Score): steatosis (0 to 3), lobular inflammation (0 to 3), ballooning (0 to 2) Cenicriviroc: oral dual antagonist of CCR-2 and CCR-5 Objective Primary: hepatic histologic improvement at year 1 ( 2 point in NAS with 1 point in lobular inflammation or ballooning) with no worsening of fibrosis ; superiority of CVC vs placebo, 80% power Friedman SL, Hepatology 2018;67:1754-67, Ratziu, EASL 2018, Abs. GS-002 Cenicriviroc - Phase 2b
CENTAUR Study: cenicriviroc in NASH (phase 2b) Baseline characteristics and disposition CVC, N = 145 Placebo, N = 144 Mean age, years 55 54 Female 50% 55% Mean BMI, kg/m2 33.6 34.1 Diabetes mellitus 57% 44% 3 criteria of metabolic syndrome, % 72 72 NASH CRN fibrosis stage 1 / 2 / 3 NAS Mean 5, % Mean steatosis Mean lobular inflammation Mean ballooning 32% / 29% / 39% 33% / 28% / 38% Liver histology 5.3 5.4 73.1% 1.4 2.4 1.5 75.0% 1.4 2.4 1.5 Discontinued before end of Year 1, N For adverse event Withdrew consent 20 9 10 18 8 7 Entered Year 2 of the study, N Early withdrawal, N / for adverse event, N 121 12 / 5 CVC = 61 2 /1 PCB = 60 2 /0 Friedman SL, Hepatology 2018;67:1754-67, Ratziu, EASL 2018, Abs. GS-002 Cenicriviroc - Phase 2b
CENTAUR Study: cenicriviroc in NASH (phase 2b) Primary endpoint at Year 1: improvement in NAS and no worsening of fibrosis, ITTm % 50 OR = 0.82 (0.44 - 1.52) 40 p = 0.52 30 18.8 20 15.9 10 0 Placebo N = 144 CVC N = 145 Friedman SL, Hepatology 2018;67:1754-67 Cenicriviroc - Phase 2b
CENTAUR Study: cenicriviroc in NASH (phase 2b) Key secondary endpoint at Year 1: improvement in fibrosis by 1 stage and no worsening of steato-hepatitis by baseline characteristics All patients By NAS OR = 1.2 (0.37 ; 3.9) OR = 2.92 (1.26 ; 6.78) OR = 2.20 (1.11 ; 4.35) p = 0.023 p = 0.013 % CVC Placebo p = 0.77 % 30 30 23.6 21.6 18.8 20 20 20 9.6 10.4 10 10 N = 37 32 89 94 0 0 NAS = 4 NAS > 5 By Fibrosis stage (NASH CRN) By ballooning grade OR = 2.2 (1.0 ; 4.7) OR = 1.88 (0.74 ; 4.73) % % OR = 4.1 (1.51 ; 11.2) p = 0.183 OR = 3.06 (0.74 ; 12.71) p = 0.049 OR = 1.15 (0.44 ; 3.02) p = 0.0056 40 40 OR = 3.16 (0.6 ; 16.62) p = 0.123 31.9 28.1 28 p = 0.78 30 30 p = 0.175 22.9 20 17.715.8 20 20 15.5 13.6 8.7 8.8 10 10 4.8 0 0 N = 44 Stage 1 42 35 Stage 2 34 47 Stage 3 50 82 Pooled stage 2 and 3 84 62 Grade 1 57 64 69 N = Grade 2 Friedman SL, Hepatology 2018;67:1754-67 Cenicriviroc - Phase 2b
CENTAUR Study: cenicriviroc in NASH (phase 2b) Improvement in fibrosis by 1 stage and no worsening of steato-hepatitis Favors placebo Favors CVC 29/126 (23%) 15/126 (12%) Overall mITT population NAS NAS = 4 NAS > 5 8/37 (22%) 21/89 (24%) 6/32 (19%) 9/94 (10%) Fibrosis stage (NASH CRN) Stage 1 Stage 2 Stage 3 6/44 (14%) 8/35 (23%) 15/47 (32%) 2/42 (5%) 3/34 (9%) 10/50 (20%) Hepatocellular ballooning Grade 1 (few ballon cells) 11/62 (18%) 9/57 (16%) Grade 2 (prominent ballooning) 18/64 (28%) 6/69 (9%) Lobular inflammation Grade 2 (2-4 foci/200x) Grade 3 (> 4 foci/200x) 17/66 (26%) 11/54 (20%) 7/61 (12%) 7/61 (12%) Steatosis Grade 1 (5-33 %) Grade 2 (> 33-66 %) 21/89 (24%) 8/31 (26%) 10/81 (12%) 4/41 (10%) BMI < 30 kg/m > 30 - < 35 kg/m > 35 - < 40 kg/m > 40 kg/m 7/32 (22%) 8/51 (16%) 6/22 (27%) 7/19 (37%) 3/34 (9%) 5/45 (11%) 4/28 (14%) 3/19 (16%) Type 2 diabetes status Present 17/74 (23%) 10/57 (18%) Not present 15/52 (23%) 5/69 (7%) Friedman SL, Hepatology 2018;67:1754-67 Cenicriviroc - Phase 2b
CENTAUR Study: cenicriviroc in NASH (phase 2b) Change in liver biopsy at year 1 (IPP population) Placebo N = 123 CVC N = 123 Improved No change Worsened Improved No change Worsened 74.0 61.8 Steatosis 19.5 6.5 25.2 13.0 43.9 49.6 Lobular inflammation 31.7 24.4 27.6 22.8 54.5 50.4 Ballooning 26.8 18.7 35.0 14.6 Biomarkers of inflammation Marked reductions in circulating biomarkers of systemic inflammation (high-sensitivity C-reactive protein, interleukin-6, interleukin-8, fibrinogen, and IL-1 ) and of monocyte activation (sCD14) were observed with CVC (vs placebo). Persistence of systmeic anti-inflamamtory activity at Year 2, without no metabolic disturbances Friedman SL, Hepatology 2018;67:1754-67, Ratziu, EASL 2018, Abs. GS-002 Cenicriviroc - Phase 2b
CENTAUR Study: cenicriviroc in NASH (phase 2b) Main outcomes at Year 2 Improvement in fibrosis ( 1 stage) Improvement in fibrosis ( 1 or 2 stages) and no worsening of NASH Maintenance of antifibrotic response from Year 1 to Year 2 (groups with 2 years CVC vs 2 years of placebo) Effects of 2 years of CVC treatment (groups with 2 years CVC vs 2 years of placebo) Effects of 1 year of CVC treatment (immediate or deferred CVC vs placebo) Safety and tolerability of CVC vs placebo during Year 2 Ratziu, EASL 2018, Abs. GS-002 Cenicriviroc - Phase 2b
CENTAUR Study: cenicriviroc in NASH (phase 2b) Antifibrotic response at year 2, ITTm CVC Placebo % 40 p = 0.63 30 26 p = 0.94 22 p = 0.13 20 15 17 11 10 3 0 N evaluable = N enrolled = 99 145 54 72 99 145 54 72 65 86 Stage 2 or 3 Improvement in fibrosis by 2 stages and no worsening of NASH 34 50 Improvement in fibrosis by 1 stage Improvement in fibrosis by 1 stage and no worsening of NASH Ratziu, EASL 2018, Abs. GS-002 Cenicriviroc - Phase 2b
CENTAUR Study: cenicriviroc in NASH (phase 2b) Adverse events and laboratory abnormalities, % CVC 1styear N = 144 Placebo 1styear N = 145 CVC 2ndyear N = 182 Grade 3-4 drug-related adverse event 8.3 6.3 12.1 Serious adverse event 11.1 6.9 NA Discontinuation for adverse event 6.3 6.9 2.7 Most common adverse events of grade 2 Fatigue Diarrhea Headache ALT increase grade 2 2.8 2.1 1.4 NA 0.7 0.7 3.5 NA NA NA NA 7.1 Grade 3-4 laboratory abnormalities Fasting serum glucose > 250 mg/dL ALT > 5 x ULN AST > 5 x ULN Triglycerides grade 3 / grade 4 Gamma-GT grade 3 / grade 4 Creatine kinase grade 3 / grade 4 Uric acid grade 3 / grade 4 Amylase > 2-5 x ULN Phosphorus < 2-1 mg/dL Absolute neutrophil grade 3 / grade 4 11.9 11.8 4.9 3.5 / 2.1 5.6 / 0.7 4.2 / 1.4 6.3 / 7.6 4.2 3.5 1.4 / 1.4 9.2 11.8 6.9 4.9 / 2.1 4.2 / 0.7 4.9 / 1.4 6.3 / 4.2 0.7 1.4 2.1 / 0.7 NA Friedman SL, Hepatology 2018;67:1754-67, Ratziu, EASL 2018, Abs. GS-002 Cenicriviroc - Phase 2b
CENTAUR Study: cenicriviroc in NASH (phase 2b) Summary CVC showed a significant antifibrotic benefit at year 1 and was well tolerated Although the primary endpoint of the study was not met, the fact that the CENTAUR year 1 study results showed that CVC provided clinically meaningful benefits and resulted in twice as many subjects achieving improvement in fibrosis by 1 stage and no worsening of steato-hepatitis as compared to placebo suggests that the study did, in fact, show proof of concept, warranting phase 3 development of CVC Year 2 analyses corroborate CVC antifibrotic activity in adults with NADH and liver fibrosis Effect more pronounced in stage 3 fibrosis Friedman SL, Hepatology 2018;67:1754-67, Ratziu, EASL 2018, Abs. GS-002 Cenicriviroc - Phase 2b