BRAAVE 2020 Study: Week 72 Outcomes and COVID-19 Impact in Black American Adults with HIV
The BRAAVE 2020 study focused on Black American adults with HIV who were virologically suppressed and switched to B/F/TAF treatment. The study analyzed outcomes at Weeks 0, 24, 48, and 72, with an extension phase involving 330 participants. Operational challenges due to COVID-19 were addressed through virtual visits and local lab usage. Efficacy results showcased promising data, emphasizing the importance of tailored HIV treatment strategies.
Uploaded on Sep 24, 2024 | 2 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
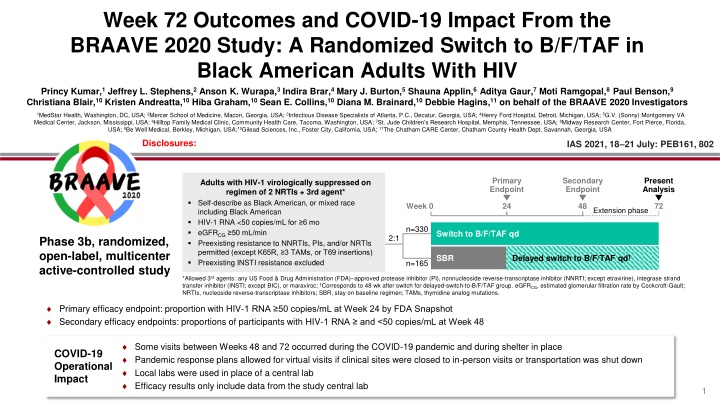
Week 72 Outcomes and COVID-19 Impact From the BRAAVE 2020 Study: A Randomized Switch to B/F/TAF in Black American Adults With HIV Princy Kumar,1 Jeffrey L. Stephens,2 Anson K. Wurapa,3 Indira Brar,4 Mary J. Burton,5 Shauna Applin,6 Aditya Gaur,7 Moti Ramgopal,8 Paul Benson,9 Christiana Blair,10 Kristen Andreatta,10 Hiba Graham,10 Sean E. Collins,10 Diana M. Brainard,10 Debbie Hagins,11 on behalf of the BRAAVE 2020 Investigators 1MedStar Health, Washington, DC, USA; 2Mercer School of Medicine, Macon, Georgia, USA; 3Infectious Disease Specialists of Atlanta, P.C., Decatur, Georgia, USA; 4Henry Ford Hospital, Detroit, Michigan, USA; 5G.V. (Sonny) Montgomery VA Medical Center, Jackson, Mississippi, USA; 6Hilltop Family Medical Clinic, Community Health Care, Tacoma, Washington, USA; 7St. Jude Children s Research Hospital, Memphis, Tennessee, USA; 8Midway Research Center, Fort Pierce, Florida, USA; 9Be Well Medical, Berkley, Michigan, USA;10Gilead Sciences, Inc., Foster City, California, USA; 11The Chatham CARE Center, Chatham County Health Dept, Savannah, Georgia, USA Disclosures: IAS 2021, 18 21 July: PEB161, 802 Primary Endpoint Secondary Endpoint Present Analysis Adults with HIV-1 virologically suppressed on regimen of 2 NRTIs + 3rd agent* Self-describe as Black American, or mixed race including Black American HIV-1 RNA <50 copies/mL for 6 mo eGFRCG 50 mL/min Preexisting resistance to NNRTIs, PIs, and/or NRTIs permitted (except K65R, 3 TAMs, or T69 insertions) Preexisting INSTI resistance excluded Week 0 24 48 72 Extension phase n=330 Switch to B/F/TAF qd 2:1 Phase 3b, randomized, open-label, multicenter active-controlled study SBR Delayed switch to B/F/TAF qd n=165 *Allowed 3rdagents: any US Food & Drug Administration (FDA) approved protease inhibitor (PI), nonnucleoside reverse-transcriptase inhibitor (NNRTI; except etravirine), integrase strand transfer inhibitor (INSTI; except BIC), or maraviroc; Corresponds to 48 wk after switch for delayed-switch-to-B/F/TAF group. eGFRCG, estimated glomerular filtration rate by Cockcroft-Gault; NRTIs, nucleoside reverse-transcriptase inhibitors; SBR, stay on baseline regimen; TAMs, thymidine analog mutations. Primary efficacy endpoint: proportion with HIV-1 RNA 50 copies/mL at Week 24 by FDA Snapshot Secondary efficacy endpoints: proportions of participants with HIV-1 RNA and <50 copies/mL at Week 48 Some visits between Weeks 48 and 72 occurred during the COVID-19 pandemic and during shelter in place Pandemic response plans allowed for virtual visits if clinical sites were closed to in-person visits or transportation was shut down Local labs were used in place of a central lab Efficacy results only include data from the study central lab COVID-19 Operational Impact 1
Results Participant Disposition Baseline Characteristics B/F/TAF: n=330 49 (18 79) 31 96 / 2 / 2 SBR: n=165 49 (19 70) 33 96 / 4 / 0 Screened: N=558 Median age, y (range) Female at birth, % Cisgender / Transgender / Other identity, % Sexual orientation, % Heterosexual, female / male at birth Gay or bisexual, female / male at birth Hispanic/Latinx ethnicity, % Median CD4 count, cells/ L (Q1, Q3) Median eGFRCG, mL/min (Q1, Q3) Median weight, kg (Q1, Q3) Median body mass index, kg/m2 (Q1, Q3) Hepatitis B coinfection, % Baseline NRTI backbone F/TAF / F/TDF ABC/3TC Other Baseline 3rd agent INSTI / NNRTI / PI / CCR5 antagonist Baseline ARV resistance NRTI resistance M184V/I NNRTI resistance PI resistance Screen failures: n=54 Not randomized: n=8* B/F/TAF randomized, not treated: n=1 Randomized: N=496 29 F / 19 M 1 F / 49 M 5 747 (570, 922) 110 (88, 132) 88 (79, 103) 29.2 (25.9, 34.0) 5 32 F / 25 M 2 F / 41 M 3 758 (494, 969) 107 (86, 132) 89 (76, 104) 29.3 (25.7, 34.3) 2 B/F/TAF treated n=330 SBR treated n=165 n=10 Prematurely discontinued n=2 Delayed B/F/TAF switch at Week 24, n=163 Completed 24 wk treatment n=320 68 / 17 13 1 65 / 21 15 0 n=15 Prematurely discontinued n=7 4 AE 2 6 Lost to follow-up 1 61 / 30 / 9 / 0 60 / 31 / 8 / 1 3 Participant decision 3 1 Investigator discretion 1 13 9 21 11 16 12 19 15 1 Death 0 Completed study drug: B/F/TAF, n=305; delayed switch to B/F/TAF, n=156 Total: n=461 Includes: INSTIs dolutegravir, elvitegravir, and raltegravir; NNRTIs doravirine, efavirenz, etravirine, nevirapine, and rilpivirine; PIs ritonavir (r) and cobicistat (c) boosted darunavir, atazanavir/c, and unboosted atazanavir; lopinavir/r and nelfinavir; and CCR5 antagonist maraviroc; Assessed by cumulative historical or retrospective baseline proviral DNA genotypes; 3TC, lamivudine; ABC, abacavir; ARV, antiretroviral; CD4, cluster of differentiation-4; TDF, tenofovir disoproxil fumarate; Q, quartile. *Met all eligibility criteria, but were not randomized: withdrew consent (n=3), lost to follow-up (n=3), outside of visit window (n=1), and other (n=1); Due to gunshot. 2
Virologic Outcome Through Week 72 B/F/TAF Total Population: HIV-1 RNA <50 Copies/mL, Missing=Excluded, Full-Analysis Set Delayed switch to B/F/TAF* 100 100 100 100 100 99 99 99 99 99 99 99 98 98 100 80 Participants, % 60 40 20 n= 328 163 323 161 326 159 323 159 320 150 316 127 277 248 0 72 0 4 12 24 36 48 60 72 60 B/F/TAF Start Weeks After Switch to B/F/TAF By Resistance Subgroups : HIV-1 RNA <50 Copies/mL at Last Visit 100 100 100 100 99 99 99 99 100 80 Participants, % 60 40 No treatment-emergent resistance was detected in any treatment group 20 43 43 25 25 267 269 130 131 30 30 20 20 280 282 135 136 0 Yes No Yes No *Study Week 72 corresponds to 48 wk after switch; Denominator for percentage is no. of participants in B/F/TAF full-analysis set with nonmissing HIV-1 RNA value at each visit; Includes participants with 1 on-treatment HIV-1 RNA measurement and baseline resistance assessed by cumulative historical or proviral DNA genotypes: B/F/TAF, n=312; SBR, n=156. NRTI Resistance M184V or M184I Mutation 3
Adverse Events and Abnormal Laboratory Values Sex at Birth Female n=156 78 10 1 5 6 3 6 6 6 7 5 4 3 Age Male n=337 79 8 8 6 5 6 4 4 4 1 5 5 4 <50 y n=254 81 9 9 4 6 7 4 4 4 4 6 6 6 50 y n=239 77 8 2 7 5 3 6 5 5 3 4 4 3 % Any AE URTI Syphilis Pain in extremity Headache Nasopharyngitis Arthralgia Cough Chest pain Urinary tract infection Hypertension Diarrhea Back pain B/F/TAF n=330 72 (71.4, 72.3) Delayed Switch n=163 48.0 (47.3, 48.3) Median study drug exposure, wk (Q1, Q3) All-grade AEs (>5% in any subgroup) All B/F/TAF n=493* 9 6 6 6 5 5 5 All Participants Who Received B/F/TAF at Any Time, % Upper respiratory tract infection Syphilis Headache Pain in extremity Arthralgia Hypertension Nasopharyngitis All-Grade AEs ( 5%) Total AEs leading to study drug discontinuation: n=12 of 493 Diarrhea Nightmare Headache Diarrhea, dry mouth, psychomotor hyperactivity, agitation, anxiety, insomnia Migraine Acute kidney injury (secondary to obstruction) Abdominal distention and flatulence Headache and hyperhidrosis Hemorrhage of intracranial aneurysm with multiple sequelae Change of bowel consistency and flatulence COVID-19 COVID-19 Nonfasting hyperglycemia Glycosuria Fasting LDL increased Fasting hyperglycemia Urine RBC (hematuria: quantitative or dipstick) 4 5 3 3 3 Grade 3 or 4 Lab Abnormalities ( 2%) *Includes all participants in B/F/TAF and delayed-switch groups; Occurred in participants with medical diagnosis of diabetes; Each in women during menses; Includes all treated with 1 dose of B/F/TAF; each row represents 1 participant; Reported as treatment related by investigator. LDL, low-density lipoprotein; RBC, red blood cells. After Week 48 4
B/F/TAF Week 72 Delayed switch to B/F/TAF Week 48 Fasting Lipids* and Weight Change B/F/TAF SBR Delayed switch to B/F/TAF* Total LDL HDL Total Cholesterol: HDL Median Change From Baseline Cholesterol Cholesterol Cholesterol Triglycerides 1 15 Median Change From 10 Baseline, mg/dL 0.5 5 0.1 1 0.1 0 0 -2 -5 -3 -3 -3 -3 -4 -0.5 -10 -9 -1 -15 Baseline, mg/dL 181 169 111 102 54 53 98 95 3.3 3.1 Weight Change: All B/F/TAF Weight Change: Randomized Treatment Arm From Baseline, kg (Q1, Q3) 6 6 Median Weight Change p=0.09 4 4 1.8 2 2 0.9 0.9 0.9 0.9 0.6 0 0 0.2 -2 -2 0 12 24 0 12 24 36 48 60 72 4 4 Week 318 Week 325 163 320 164 B/F/TAF: n= SBR: n= 330 165 330 325 320 315 277 229 163 159 158 149 119 Delayed switch: n= *Baseline is median value at time of 1st B/F/TAF dose; Study Week 72 for B/F/TAF corresponds to 48 wk after switch. From 2-sided Wilcoxon rank-sum test comparing B/F/TAF vs SBR at Week 24. HDL, high-density lipoprotein. 5
COVID-19 Impact on Study Participation 30 27 B/F/TAF Delayed switch 24 Participants, % 20 15 14 12 8 10 2 2 0.6 0.6 0.6 0.3 0.3 0 0 1 2 1 2 3 1 1 Missed Visits Virtual Visits Participants had high study engagement with few missed visits despite the COVID-19 pandemic 124 participants (25%) completed virtual visits in lieu of site visits 6 participants (1%) missed visits (in person and/or virtual) due to COVID-19-related challenges The last participant visit was 19 Aug 2020 5 participants were reported to have COVID-19 and 2 died 6
Conclusions For Black Americans living with HIV, switching to B/F/TAF was highly effective and safe through 72 weeks, regardless of age, sex at birth, or preexisting NRTI resistance No participant had treatment-emergent resistance to study drugs Small reductions in median changes from baseline in total cholesterol and triglycerides were observed after switching to B/F/TAF Weight changes were similar between groups at Week 24 and stable from Weeks 24 to 72 More weight gain was observed in participants switching from TDF and ABC compared with TAF Black American participants had high study engagement, with few missed visits and high adherence despite the COVID-19 pandemic 7
References 1. Daar ES, et al. Lancet HIV 2018;5:e347-56; 2. Kityo C, et al. J Acquir Immune Defic Syndr 2019;82:321-8; 3. Molina J-M, et al. Lancet HIV 2018;5:e357-65; 4. Hagins D, et al. JAIDS May 18, 2021 [epub]; 5. Saag MS, et al. JAMA 2018;320:379-96; 6. Clinical Info HIV.gov. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV; 7/10/19; 7. European AIDS Clinical Society. Guidelines Version 10.0, November 2019; 8. Sax PE, et al. Clin Infect Dis 2020 Jul 15;ciaa988; 9. AIDSVu.org. Map. 8