Biodiesel Production from Alligator Fat: Sustainable Fuel Solution
Biodiesel production from waste alligator fat offers a sustainable solution for energy security and environmental benefits. Louisiana's abundant alligator population provides a source for biofuel production, reducing reliance on fossil fuels. The process involves converting animal fats into biodiesel using supercritical methanol. With the increasing popularity of biofuels, utilizing alligator fat presents a viable alternative fuel source, contributing to economic stability and reducing waste.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
UNIVERSITY OF LOUISIANA AT LAFAYETTE RAY P. AUTHEMENT COLLEGE OF SCIENCES DEPARTMENT OF CHEMISTRY Biodiesel from alligator fat using supercritical methanol via a laboratory scale flow reactor August Gallo Thomas Junk University of Louisiana at Lafayette Department of Chemistry
Fossil fuels According to the Energy Information Administration (EIA), the world energy consumption is projected to increase by 33% from 2007 to 2035 In the year 2007 the United States alone consumed 21% of the world s energy As of 2008, transportation share of the U.S. petroleum consumption is 71% The U.S transportation sector is 97 percent reliant on oil, with 60 percent of this oil imported
Biofuels Energy security Economic stability Environmental gains Dominant biofuels- biodiesel and bioethanol. Food vs. biofuels
Alligator and its fats In United States, Louisiana has the population of about 1.5 million alligators constituting 75% of the total population Louisiana s wild and farm raised alligator harvest is approximately 275,000 per year In the year 2008 the revenue generatedfrom wild and farm raised alligators was ~ $70 million 15 million pounds of alligator fat is available annually in Louisiana, which is currently being disposed as waste The increasing popularity of alligator farming, coupled with the high fat content of alligators, strongly supports the use of waste alligator fat as a source for biodiesel fuel. It stands to reason, however, that the methods presented here are of general applicability for the conversion of other animal fats (e.g., lard, chicken fat) to biodiesel.
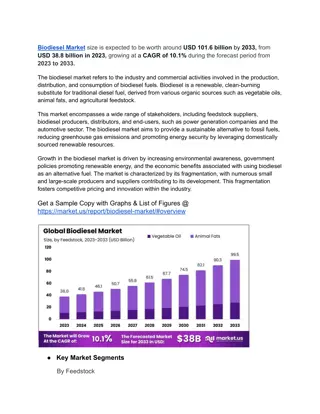
Current status Biodiesel is increasingly used as a renewable alternative to conventional diesel fuel. With approximately 51 billion liters in 2010, the United States is the largest producer of biofuels. Biodiesel is prepared from vegetable or animal fats, usually by transesterification with methanol (Fig. 1)
Traditional processing An established procedure for the transesterification of fats with methanol consists of stirring the respective fat with methanol and a suitable catalyst, usually sodium hydroxide. An alternative procedure is the rendering of oil prior to transesterification, leaving a residue of nitrogen-rich material. In each case, solid byproducts and an emulsion are obtained, which have to be separated by filtration and/or centrifugation and generate a significant amount of caustic solid waste. A typical setup is shown in Fig. 2, the formation of solids in the product mixture is evident by the turbid appearance of the fluid phase. Optimized conditions consisted of heating the fat with methanol in a 3:1 weight ratio for 2.5 hours at 65 C in the presence of 1% of NaOH.
Conversion methanol - rationale We are developing an alternative procedure with several objectives in mind: 1) the reduction of elimination of solid waste products, 2) the avoidance of emulsions and caustic residues that require additional separation steps, and 3) reduced reaction times. The use of supercritical methanol eliminates the need for a catalyst, promises short reaction times, and produces product mixtures that are virtually free of solid residues. Methanol has a critical temperature of approx. 240 C, as shown in the diagram below (Fig. 3) using supercritical
Fatty acid profile of alligator fat 1:4 MR 2 h % area (LSU) C14:0 0.69 C15:0 0.04 C16:0 15.04 C16:1 8.75 C17:0 0.08 C18:0 2.98 C18:1 30.37 C18:2 22.45 C18:3 1.92 C20:1 - C20:3 - C20:4 - C21:0 17.67 1:4 MR 2 h % area (ULL) 1.22 - 22.78 11.81 - 4.90 56.63 - - 1.56 0.58 0.53 - Fatty acids 1:4 MR 2 h % area (LSU) 1:4 MR 2 h % area (ULL) Fatty acids Unsaturated 63.5 78.11 Saturated 36.5 28.9
GC analysis Main methyl ester after treatment in flow reactor
Conclusions Batch process The use of supercritical methanol in the conversion of waste alligator fat to biodiesel was shown to be a viable alternative to traditional transesterification. Key advantages of this method include: a) avoidance of caustic catalysts, b) the elimination of solid byproducts posing disposal problems, and c) significant simplification of the conversion process, no emulsions. The use of raw fat containing proteins resulted in the formation of liquid amines and amides in the resulting product. These pose no harm; in fact, the addition of amines to increase the thermal stability of diesel fuels has been patented (USP 4,166,726). Furthermore, it is common practice to co-inject solutions of urea into diesel engines to reduce harmful NOX emissions. Optimized batch conditions were achieved when 2 g of oil was heated with 8 g of methanol to 300 C for 3 hours, resulting in 95.8 % of methyl esters and undetectable levels of free acids.
Flow Reactor Study Several inherent problems with the batch process- temperature control after completion of the reaction. With a flow reactor, the reactants can enter and leave the system under more controlled conditions. A simple system was devised using an HPLC pump, shut off valve, woods metal bath, heater, digital thermometer, 6 ft. column, water cooled jacket and a pressure valve to control the pressure.
Flow Reactor Study-FAME analysis Concentration of the main methyl ester as a function of time, 1 mL/min
Flow Reactor Study-FAME analysis Concentration of the main methyl ester as a function of time, 0.2 mL/min
Flow Reactor Study-FAME analysis Concentration of the main methyl ester as a function of temperature - 1 mL/min
Conclusions Flow Reactor These graphs show the concentration of the esters increases exponentially. The highest concentration was reached at 400 C for the all esters, then the concentrations decreased rapidly. For the higher flow rate the exponential decrease was not observed. A new run was carried at 300, 320, 340, 360, 380, 400, 420, 440, 460, 480 C and 500 C. The number of samples was increased in order to have more information about the ester concentration especially between 400 C and 500 C. These graphs show that the highest concentration was reached at 460 C. In this case we observed, for the unsaturated ester, a exponential decrease of the concentration.
Economicevaluation The economics of the lipid production was investigated with the following assumptions: The cost of lipid source was assigned to be zero, since feed stock was alligator fat waste that normally entails disposal costs for the processing industry. Byproduct glycerol was assumed to fetch a price of $ 0.22 per kg (ICIS, 2010). Cost of equipment were taken from SuperPro Designer and from the equipment data of Peters and Timmerhaus (Peter and Timmerhaus, 1981).
Equipment cost No of equipments Name of equipment Total cost Grinder 1 73,000 Electric heater 2 63,000 Heat exchanger 1 1000 Nutsche filter 1 240,000 Storage tank 1 45,000 Vessel reactor 2 129,766.8 Distillation column 1 21,296.36 Cooling heat exchanger 1 1 1273.41 Cooling heat exchanger 2 1 1297.67 Total cost 575634.24
Utilities Name of equipment Utilities Electricity KW h/y Grinder 650,240 Electric heater 1,504,512 Vessel reactor 7554.88 Cooling water/steam Cooling water 37547 m3/y Steam 581000 kg/y
Results (Economic analysis) Fixed capital investment = 2.51 million dollars. Total capital investment = 2.98 million dollars. Raw material cost = 0.44 million dollars. Labor cost (18 people) = 0.87 million dollars. Utility costs = 0.125 million dollars. Annual total product cost = 3.01 million dollars. Average return on investment = 28.5% per year. Payback period = 2.3 years.
Acknowledgements I would like to thank the department of chemistry at UL Lafayette and the following individuals who made this work possible through support and guidance: Dr. Rakesh Bajpai, Professor of Chemical Engineering, UL Dean Mark Zappi, Dean of Engineering, UL Patrick Spiller, UL Kevin Martin, University of Poitiers, France Teddy Lacourcelle, University of Poitiers, france Dr. William Holmes, UL Dr. Yuemin Liu, UL