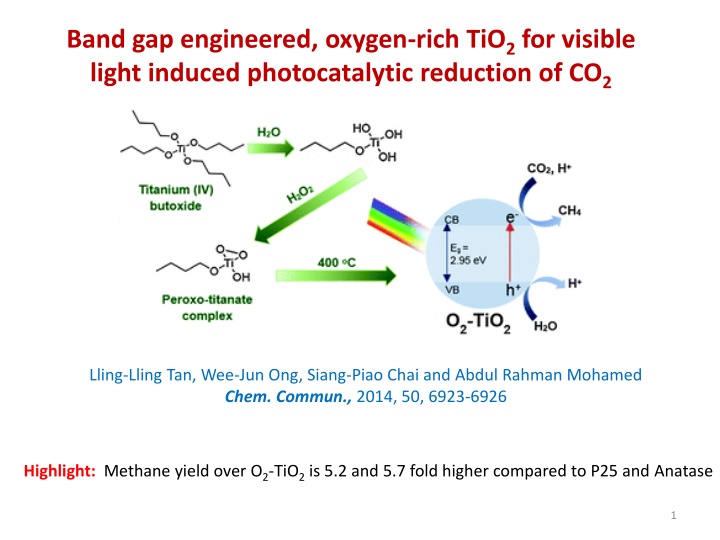
Band Gap Engineered Oxygen-Rich TiO2 for Visible Light-Induced Photocatalytic Reduction of CO2
Band gap engineering and oxygen-rich modifications in TiO2 have been explored for visible light-induced photocatalytic reduction of CO2. The engineered O2-TiO2 photocatalyst exhibited significantly higher methane yield compared to conventional TiO2 types. The preparation involves hydrolysis of titanium species, peroxo-titanate complex formation, and subsequent calcination. Characterization techniques such as FT-IR, XRD, TEM, HRTEM, and XPS confirm the structural and chemical properties of the O2-TiO2 material.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Band gap engineered, oxygen-rich TiO2 for visible light induced photocatalytic reduction of CO2 Lling-Lling Tan, Wee-Jun Ong, Siang-Piao Chai and Abdul Rahman Mohamed Chem. Commun., 2014, 50, 6923-6926 Highlight: Methane yield over O2-TiO2 is 5.2 and 5.7 fold higher compared to P25 and Anatase 1
Photocatalyst Preparation Titanium (IV) butoxide Cold water (5 oC) hydrolyzed titanium species (precipitate) repeatedly washed with water stirred with H2O2 for 1 h peroxo-titanate complex in solution An illustration for the preparation of the O2 TiO2 photocatalyst heating at 50 oC for 3 h gelation dried in an air oven at 100 oC for 24 h calcination at 400 oC for 2 h (10 oC/min) O2-TiO2 yellowish solid material 2
Infrared analysis of Peroxo-titanate complex FT-IR spectra of 100 oC dried TiO2 precursor: (a) control and (b) H2O2- modified TiO2 intermediate, (B) Enlarged spectra for wavenumber range 400-1200 cm-1. 3
XRD pattern of O2TiO2 XRD pattern of O2 TiO2 Lattice parameters of ATiO2 and O2 TiO2 4
Location of excess oxygen increase in the lattice constant a Atomic structures of (A) ATiO2 and (B) (O2)O 5
O2TiO2 Crystal phase & Particle size (A) TEM and HRTEM images of O2 TiO2 showing (B) anatase and (C) rutile TiO2, (D) Particle size distribution of O2 TiO2. 6 A local epitaxial relationship might exist between the planes of anatase (101) and rutile (110)
Commercial anatase with d= 0.352 nm TEM and (B) HRTEM images of ATiO2 7
Raman analysis of O2TiO2 Raman spectrum O2 TiO2 8
Ti4+ chemical state confirmed No trace of Ti3+ High oxygen in O2-TiO2 compared to ATiO2 (C) Ti 2p and (D) O 1s-XPS peaks of (a) ATiO2 and (b) O2 TiO2 9
(E) UV-Vis DRS of (a) ATiO2 and (b) O2TiO2, (F) The corresponding plot of the transformed KM function [F(R)h ]1/2 vs. h . 10
(A) UV-Vis DRS and (B) plot of the transformed KM function for rutile, P25, ATiO2 and O2- TiO2 11
Experimental set-up for the photocatalytic reduction of CO2 under visible light irradiation 12
Light spectrum of daylight bulb used in photocatalytic experiments Light spectrum of the daylight bulb used in the photoreduction of CO2 13
Enhancement factors of 5.7 and 5.2 over ATiO2 and P25 for methane yield were observed with O2-TiO2 Time dependence of the photocatalytic formation rate of CH4 under visible light irradiation. Control experiments performed under a N2/H2O flow and in the absence of light irradiation are also included. 14
Band gap narrowing (2.95 Vs 3.2 eV) and photocatalytic enhancement were attributed to the oxygen-rich nature of our final product Fraction of rutile phase in O2-TiO2 was close to that of P25 15
Mechanism for the photocatalytic reduction of CO2 over O2-TiO2 Schematic of visible absorption and photocatalytic mechanism in the presence of O2-TiO2 nanoparticles 16
Summary A visible-light-responsive oxygen-rich TiO2 photocatalyst was synthesized via a facile aqueous peroxo titania route. The introduction of H2O2 into the precursor led to a drastic modification of the band structure of TiO2. The O2 TiO2 demonstrated excellent performance for the photocatalytic reduction of CO2 to CH4 under visible light irradiation. Two primary factors responsible for the high visible light photoactivity of O2-TiO2 are (i) the band gap narrowing effect of the oxygen excess defect and (ii) the enhanced absorption intensity in the visible region The findings could open up a scalable and cost-effective approach to obtain robust materials for photocatalytic applications 17