Analysis of Cu Minerals Stability in Various Gas Pressures
Investigating the stability of various copper minerals (Cuprite, Tenorite, Malachite, Azurite) in relation to the partial pressures of CO2 and O2 gases. Thermodynamic data and equilibrium constants are used to determine equilibrium O2 pressures for reactions involving copper minerals and oxygen. The relationships between log PO2 values are plotted on a bar graph to identify metastable reactions and assess the stability ranges of CuO and Cu2O under different gas pressures.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
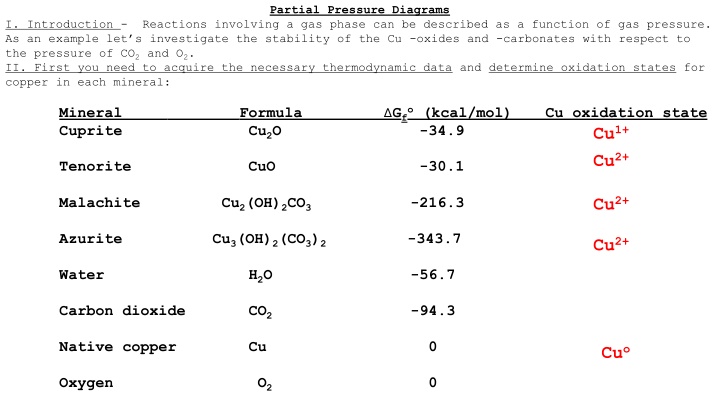
Partial Pressure Diagrams I. Introduction - Reactions involving a gas phase can be described as a function of gas pressure. As an example let s investigate the stability of the Cu -oxides and -carbonates with respect to the pressure of CO2 and O2. II. First you need to acquire the necessary thermodynamic data and determine oxidation states for copper in each mineral: Gfo (kcal/mol) -34.9 Mineral Cuprite Cu2O Formula Cu oxidation state Cu1+ Cu2+ Tenorite CuO -30.1 Cu2+ Malachite Cu2(OH)2CO3 -216.3 Azurite Cu3(OH)2(CO3)2 -343.7 Cu2+ Water H2O -56.7 Carbon dioxide CO2 -94.3 Native copper Cu 0 Cuo Oxygen O2 0
III. Write reactions that involve only Cu-minerals and oxygen being sure to include all the possible combinations: 2 Cu (c) + 1/2 O2 (g) = Cu2O (c) (1) Cu (c) + 1/2 O2 (g) = CuO (c) (2) Cu2O (c) + 1/2 O2 (g) = 2 CuO (c) (3) A. Because the activities of solids equal 1, each reaction is at equilibrium at a particular partial pressure of O2. B. Determine the equilibrium constants and, thereby, the equilibrium O2 pressures for these reactions using the following relationship: G r log K = --------- -1.364
C. Reactions involving only copper and oxygen 1. Reaction (1) (Native copper to cuprite) 2 Cu (c) + 1/2 O2 (g) = Cu2O (c) (1) GR = -34.9 - [2 (0) + 1/2 (0)] = -34.9 kcal/mol log K = 25.6 [Cu2O] K = ------------------ = 1025.6 [Cu]2 [O2]0.5 log K = log[Cu2O] - 2 log[Cu] - 1/2log[PO2] = 25.6 log K = log[1] - 2 log[1] - 1/2log[PO2] = 25.6 log K = 0 - 0 - 1/2log[PO2] = 25.6 log K = - 1/2log[PO2] = 25.6 log[PO2] = -51.2
2. Reaction (2) (Native copper to tenorite) Cu (c) + 1/2 O2 (g) = CuO (c) (2) GR = -30.1 - [(0) + 1/2 (0)] = -30.1 kcal/mol log K = -1/2 log P O2 = 22.1 log P O2 = -44.1 3. Reaction (3) (Cuprite to tenorite) Cu2O (c) + 1/2 O2 (g) = 2 CuO (c) (3) GR = 2(-30.1) - [(-34.9) + 1/2 (0)] = -25.3 kcal/mol log K = -1/2 log P O2 = 18.5 log P O2 = -37.1
D. Display relationships on bar graph of log PO2 to check for any metastable reactions. -30 --------------- L o g P O 2 -40 --- CuO -37.1 Cu2O CuO -44.1 Cuo -50 --- Cuo Cu2O -51.2 -60 --------------- -51.2 -37.1 What is the maximum log PO2 at which Cuo is stable? What is the minimum log PO2 at which CuO is stable? Can Cuo & CuO exist between log PO2 values of -37.1 & -51.2? No The Cuo/CuO reaction is metastable because neither Cuo nor CuO is stable in the PO2 range in which this transition would occur (i.e., -51.2 < log PO2 < -37.1). Native copper can not exist at such a high PO2 and CuO can t exist at such a low PO2. Therefore, this reaction can never actually take place even though its equilibrium PO2 can be calculated. So, this metastable reaction can be eliminated from the diagram.
IV. Write the reactions that include only CO2 gas. A. Malachite to azurite 3 Cu2(OH)2CO3 (c) + CO2 (g) = 2 Cu3(OH)2(CO3)2 (c) + H2O (l) (4) Gr = [2(-343.7)+(-56.7)-[3(-216.3)+(-94.3)] = -0.9 kcal/mol log K = 0.66 = - log [PCO2] B. Tenorite to malachite PCO2 = 10 -0.66 2 CuO(c) + H2O (l) + CO2 (g) = Cu2(OH)2CO3 (c) (5) Gr = [-216.3] - [2(-30.1) + (-94.3) + (-56.7)] = -5.1 kcal/mol log K = 3.7 = - log [PCO2] PCO2 = 10-3.7 C. Tenorite to azurite 3 CuO(c) + H2O (l) + 2 CO2 (g) = Cu3(OH)2(CO3)2(c) (6) Gr = [-343.7] - [3(-30.1) + 2(-94.3) + (-56.7)] = -8.1 kcal/mol log K = 5.9 = - 2 (log [PCO2]) PCO2 = 10 -3.0
IV. Now write the reactions that involve both CO2 and O2 A. Cuprite to malachite Cu2O (c) + 1/2 O2 (g) + CO2 (g) + H2O (l) = Cu2(OH)2CO3 (c) (7) Gr = -30.4 kcal.mol , therefore, log K = 22.3 1 K = ------------------ (P O2)0.5 (P CO2) log K = 22.3 = -1/2 log PO2 - log P CO2 log PO2 = -44.6 - 2 log PCO2 B. Cuprite to azurite 3Cu2O(c) + 3/2O2(g) + 4CO2(g) + 2H2O(l)=2Cu3(OH)2(CO3)2(c) (8) log PO2 = -45.1 - 2.67 log PCO2 C. Native copper to malachite (9) log PO2 = -47.9 - log PCO2 D. Native copper to azurite (10) log PO2 = -48.1 - 1.33 log PCO2
Now substitute in values for logs of carbon-dioxide and oxygen pressures and plot each of the reactions on a graph such as this. Remember there are only 9 to plot because tenorite- native copper was meta- stable and does not appear on the final diagram.
Now begin eliminating other metastable reactions and the metastable extensions of reactions that can occur under some conditions but not in others. Tenorite/azurite is a metastable reaction because those two minerals can not exist in the stability field of malachite. Tenorite/malachite can not exist below the stability field of tenorite Malachite/cuprite & azurite/cuprite can t exist outside the cuprite field Tenorite/cuprite can not exist outside the tenorite stability field. Cuprite/azurite can not exist in the malachite stability field. Malachite/azurite can not exist in the cuprite or native copper stability fields Malachite/cuprite can not exist in the azurite stability field. Malachite/native copper and azurite/native copper can not exist in the cuprite stability field. Malachite/native copper can not exist in the azurite stability field. Native copper/cuprite can not exist in the azurite stability field. Don t worry, your problem set will only include a single carbonate mineral so this process will be much easier.
What is the value of log PO2in Earths atmosphere (PO2=0.21atm)? -0.68 Where does this value plot? up off graph This is tens of orders of magnitude greater than the Cuo or cuprite stability fields. Which minerals could be stable at the surface? tenorite, malachite, or azurite What determines which is stable? PCO2 What is the value of log PCO2 in Earth s atmosphere (PCO2=0.0004atm)? Which minerals are stable at this PCO2? tenorite or malachite log P O2 -3.4 Note that atmospheric PCO2 occurs close to the tenorite/malachite boundary. So, when Cuois exposed to Earth s atmosphere, it oxidizes extremely rapidly to form tenorite giving Cu objects a black color It then usually turns green over time as malachite forms. log P CO2 If Cl- or SO42- are present Cu chlorides & sulfates can form.