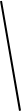Alkanes and Alkenes in Crude Oil
The process of fractional distillation separates hydrocarbons in crude oil into fractions like methane, ethane, propane, and butane. These alkanes and alkenes play a key role in producing fuels and petrochemicals, with properties and uses varying based on chain lengths and reactivity. Learn about the importance of fractional distillation, cracking processes, combustion reactions, and applications of these hydrocarbons in various industries.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
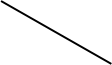
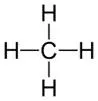
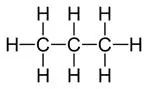
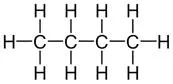
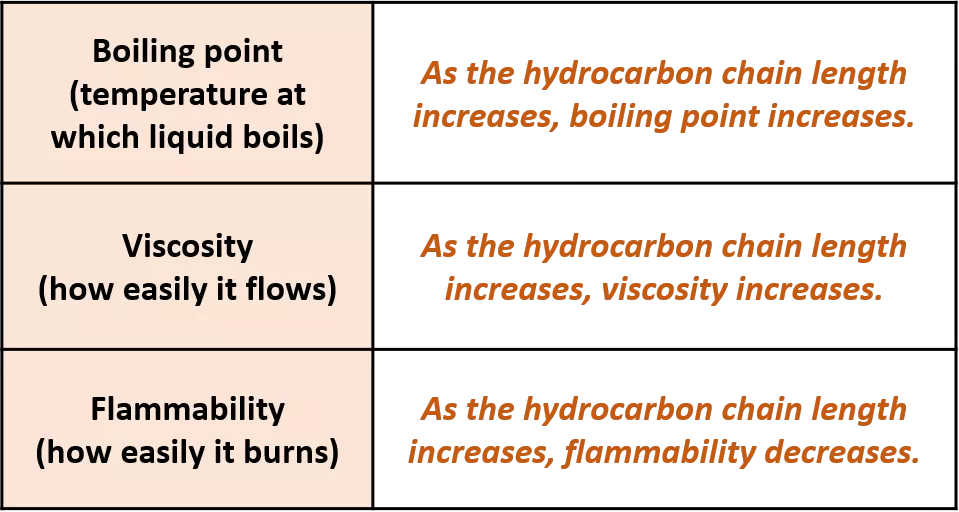
Display formula for first four alkanes Each fraction contains molecules with a similar number of carbon atoms in them. The process used to do this is called fractional distillation. Crude oil, hydrocarbons The hydrocarbons in crude oil can be split into fractions Consisting mainly of plankton that was buried in the mud, crude oil is the remains of ancient biomass. Fractions and alkanes Crude oil A finite resource Methane (CH4) Ethane (C2H6) We depend on many of these fuels; petrol, diesel and kerosene. Fractions can be processed to produce fuels and feedstock for petrochemical industry These make up the majority of the compounds in crude oil Most of these hydrocarbons are called alkanes. Using fractions Propane (C3H8) Butane (C4H10) Hydrocarbons Many useful materials are made by the petrochemical industry; solvents, lubricants and polymers. Carbon compounds as fuels and feedstock For example: General formula for alkanes CnH2n+2 C2H6 Fractional distillation and petrochemicals AQA GCSE Organic chemistry 1 C6H14 In oil Hydrocarbon chains in crude oil come in lots of different lengths. Hydrocarbon chains Alkanes to alkenes Long chain alkanes are cracked into short chain alkenes. Carbon compounds as fuels and feedstock Boiling points The boiling point of the chain depends on its length. During fractional distillation, they boil and separate at different temperatures due to this. Alkenes are hydrocarbons with a double bond (some are formed during the cracking process). Alkenes Properties of hydrocarbons Alkenes are more reactive that alkanes and react with bromine water. Bromine water changes from orange to colourless in the presence of alkenes. Cracking and alkenes Properties of alkenes During the complete combustion of hydrocarbons, the carbon and hydrogen in the fuels are oxidised, releasing carbon dioxide, water and energy. Combustion The smaller chains are more useful. Cracking can be done by various methods including catalytic cracking and steam cracking. The breaking down of long chain hydrocarbons into smaller chains Decane pentane + propene + ethane Complete combustion of methane: Methane + oxygen carbon dioxide + water + energy CH4 (g) + 2O2 (g) CO2 (g) + 2 H2O (l) Cracking C10H22 C5H12 + C3H6 + C2H4 Used to produce polymers. They are also used as the starting materials of many other chemicals, such as alcohol, plastics and detergents. Boiling point (temperature at which liquid boils) As the hydrocarbon chain length increases, boiling point increases. Alkenes and uses as polymers After vaporisation, the vapour is passed over a hot catalyst forming smaller, more useful hydrocarbons. The heavy fraction is heated until vaporised Catalytic cracking Viscosity As the hydrocarbon chain length increases, viscosity increases. (how easily it flows) After vaporisation, the vapour is mixed with steam and heated to a very high temperature forming smaller, more useful hydrocarbons. Without cracking, many of the long hydrocarbons would be wasted as there is not much demand for these as for the shorter chains. Why do we crack long chains? The heavy fraction is heated until vaporised Steam cracking Flammability (how easily it burns) As the hydrocarbon chain length increases, flammability decreases. better hope brighter future
Display formula for first four alkanes Each fraction contains molecules with a similar number of carbon atoms in them. The process used to do this is called fractional distillation. Crude oil, hydrocarbons The hydrocarbons in crude oil can be split into fractions Consisting mainly of plankton that was buried in the mud, crude oil is the remains of ancient biomass. and alkanes A finite resource Methane (CH4) Ethane (C2H6) We depend on many of these fuels; petrol, diesel and kerosene. Fractions can be processed to produce fuels and feedstock for petrochemical industry These make up the majority of the compounds in crude oil Most of these hydrocarbons are called alkanes. Propane (C3H8) Butane (C4H10) Many useful materials are made by the petrochemical industry; solvents, lubricants and polymers. Carbon compounds as fuels and feedstock For example: CnH2n+2 C2H6 Fractional distillation and petrochemicals AQA GCSE Organic chemistry 1 C6H14 Hydrocarbon chains in crude oil come in lots of different lengths. Long chain alkanes are cracked into short chain alkenes. Carbon compounds as fuels and feedstock The boiling point of the chain depends on its length. During fractional distillation, they boil and separate at different temperatures due to this. Alkenes are hydrocarbons with a double bond (some are formed during the cracking process). Properties of hydrocarbons Alkenes are more reactive that alkanes and react with bromine water. Bromine water changes from orange to colourless in the presence of alkenes. Cracking and alkenes During the complete combustion of hydrocarbons, the carbon and hydrogen in the fuels are oxidised, releasing carbon dioxide, water and energy. The smaller chains are more useful. Cracking can be done by various methods including catalytic cracking and steam cracking. The breaking down of long chain hydrocarbons into smaller chains Decane pentane + propene + ethane Complete combustion of methane: Methane + oxygen carbon dioxide + water + energy CH4 (g) + 2O2 (g) CO2 (g) + 2 H2O (l) C10H22 C5H12 + C3H6 + C2H4 Used to produce polymers. They are also used as the starting materials of many other chemicals, such as alcohol, plastics and detergents. As the hydrocarbon chain length increases, boiling point increases. After vaporisation, the vapour is passed over a hot catalyst forming smaller, more useful hydrocarbons. The heavy fraction is heated until vaporised As the hydrocarbon chain length increases, viscosity increases. After vaporisation, the vapour is mixed with steam and heated to a very high temperature forming smaller, more useful hydrocarbons. Without cracking, many of the long hydrocarbons would be wasted as there is not much demand for these as for the shorter chains. The heavy fraction is heated until vaporised As the hydrocarbon chain length increases, flammability decreases. better hope brighter future
Display formula for first four alkanes Each fraction contains molecules with a similar number of carbon atoms in them. The process used to do this is called fractional distillation. Crude oil, hydrocarbons Consisting mainly of plankton that was buried in the mud, crude oil is the remains of ancient biomass. Fractions and alkanes Crude oil Methane (CH4) Ethane (C2H6) We depend on many of these fuels; petrol, diesel and kerosene. Most of these hydrocarbons are called alkanes. Using fractions Propane (C3H8) Butane (C4H10) Hydrocarbons Many useful materials are made by the petrochemical industry; solvents, lubricants and polymers. Carbon compounds as fuels and feedstock For example: General formula for alkanes C2H6 Fractional distillation and petrochemicals AQA GCSE Organic chemistry 1 C6H14 In oil Hydrocarbon chains Alkanes to alkenes Carbon compounds as fuels and feedstock Boiling points Alkenes Properties of hydrocarbons Cracking and alkenes Properties of alkenes Combustion The smaller chains are more useful. Cracking can be done by various methods including catalytic cracking and steam cracking. Decane pentane + propene + ethane Complete combustion of methane: Methane + oxygen carbon dioxide + water + energy CH4 (g) + 2O2 (g) CO2 (g) + 2 H2O (l) Cracking C10H22 C5H12 + C3H6 + C2H4 Boiling point (temperature at which liquid boils) Alkenes and uses as polymers After vaporisation, the vapour is passed over a hot catalyst forming smaller, more useful hydrocarbons. Catalytic cracking Viscosity (how easily it flows) After vaporisation, the vapour is mixed with steam and heated to a very high temperature forming smaller, more useful hydrocarbons. Why do we crack long chains? Steam cracking Flammability (how easily it burns) better hope brighter future
Display formula for first four alkanes Crude oil, hydrocarbons Fractions and alkanes Crude oil Methane (CH4) Ethane (C2H6) Using fractions Propane (C3H8) Butane (C4H10) Hydrocarbons Carbon compounds as fuels and feedstock For example: General formula for alkanes Fractional distillation and petrochemicals AQA GCSE Organic chemistry 1 In oil Hydrocarbon chains Alkanes to alkenes Carbon compounds as fuels and feedstock Boiling points Alkenes Properties of hydrocarbons Cracking and alkenes Properties of alkenes Combustion Decane pentane + propene + ethane Complete combustion of methane: Word equation: Symbol equation: Cracking C10H22 C5H12 + C3H6 + C2H4 Boiling point (temperature at which liquid boils) Alkenes and uses as polymers Catalytic cracking Viscosity (how easily it flows) Why do we crack long chains? Steam cracking Flammability (how easily it burns) better hope brighter future