SGLT-2 inhibitors and AKI
Consultant Nephrologist Paraskevi Liaveri discusses the association between SGLT-2 inhibitors and acute kidney injury (AKI). Pharmacovigilance reports highlight a higher risk of AKI with these drugs, especially when combined with RAAS blockers, diuretics, or NSAIDs. Studies suggest potential mechani
0 views • 49 slides
Understanding Adverse Events Following Immunization (AEFI)
Adverse Events Following Immunization (AEFI) are medical incidents that occur after immunization, potentially caused by the vaccine, leading to unfavorable symptoms. Pharmacovigilance plays a crucial role in detecting, assessing, and preventing these events. AEFI can impact immunization programs at
2 views • 40 slides
Pharmacovigilance Indicators for Monitoring Health Interventions in India
The pharmacovigilance indicators outlined by the Indian Pharmacopoeia Commission focus on evaluating the status of pharmacovigilance systems in India. These indicators help measure the effectiveness of health services and interventions, identify strengths and weaknesses, and determine the impact and
2 views • 14 slides
Understanding Post-Authorisation Safety Studies (PASS) in Pharmacovigilance
Post-Authorisation Safety Studies (PASS) are conducted after a medicinal product is authorized to gather more safety information or assess risk management effectiveness. These studies evaluate the safety and benefit-risk profile of the product, aiding regulatory decision-making. PASS aims to identif
0 views • 4 slides
Understanding Medication Errors in Pharmacovigilance Centers
Adverse drug reactions resulting from medication errors are a significant concern faced by pharmacovigilance centers. This content discusses the importance of this problem, contributing factors, the relevance to pharmacovigilance centers, and strategies for managing medication errors. It highlights
4 views • 29 slides
Understanding Periodic Safety Update Reports (PSURs) in Pharmacovigilance
Periodic Safety Update Reports (PSURs) are essential pharmacovigilance documents that evaluate the benefit-risk balance of medicinal products post-authorization. These reports are submitted by Market Authorization Holders (MAH) at specified intervals to ensure the ongoing safety and efficacy of medi
3 views • 5 slides
The CLEAR Trial: Investigating Hypertonic Saline and Carbocisteine in Bronchiectasis
The CLEAR Trial is a 2x2 factorial randomized trial evaluating the effectiveness of hypertonic saline and carbocisteine for airway clearance in bronchiectasis over 52 weeks. Led by Professor Stuart Elborn and Professor Judy Bradley from Queen's University Belfast, the trial focuses on clinical and c
1 views • 23 slides
Understanding Pharmacovigilance: Importance and History
Delve into the world of pharmacovigilance through this insightful presentation covering topics such as the need for pharmacovigilance, risk/benefit balance of medications, WHO definition, detection of adverse effects, and learning from past disasters like the Thalidomide case. Explore why pharmacovi
0 views • 33 slides
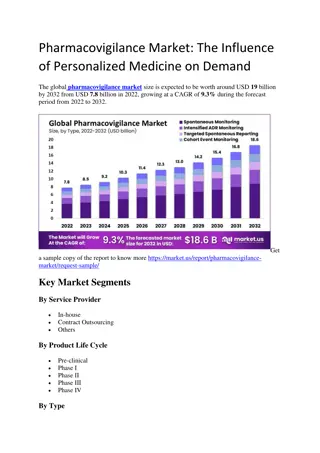
Pharmacovigilance Market
The global pharmacovigilance market size is expected to be worth around USD 19 billion by 2032 from USD 7.8 billion in 2022, growing at a CAGR of 9.3% during the forecast period from 2022 to 2032.
0 views • 4 slides
Understanding Targeted Clinical Investigation in Pharmacovigilance
Targeted clinical investigation plays a crucial role in pharmacovigilance by further evaluating significant risks identified in pre-approval clinical trials. This involves conducting pharmacokinetic and pharmacodynamic studies, genetic testing, interaction studies, and large simplified trials to ass
0 views • 12 slides
PV-Trend: A JSL Application for Pharmacovigilance Trending Topics
Application PV-Trend is designed for monitoring the safety of medicinal products, providing a user-friendly interface for defining topics, intervals, and exposures. It allows medical reviewers to create trending datasets and graphs, with statistical interpretations and significant time savings. The
0 views • 12 slides
Pharmacovigilance Guidelines for Clinical Trials in Bronchiectasis Research
The CLEAR Trial is a 2x2 factorial randomized trial evaluating the effectiveness of hypertonic saline and carbocisteine for airway clearance in bronchiectasis patients. The trial, led by Professor Stuart Elborn and Professor Judy Bradley, focuses on pharmacovigilance activities, adverse event defini
0 views • 20 slides
Pharmacovigilance Market to be Worth $18.05 Billion by 2031
The incidence of ADRs varies and depends on factors such as age, sex, ethnicity, existing disorders, genetic and environmental factors, administration route, treatment duration, dosage, and drug type. Moreover, increased age and polypharmacy also lea
0 views • 4 slides