Switch to LPV/r + RAL Study: Efficacy and Safety Findings
This study, named KITE, focused on switching to LPV/r and RAL treatment in HIV patients without virologic failure. The trial included 40 participants continuing triple therapy, with primary endpoints being HIV RNA levels and event-free treatment failure. Results showed high virological response rate
0 views • 6 slides
CEOS LPV Fire Disturbance Products Overview
CEOS LPV Fire Disturbance products play a crucial role in monitoring and validating active fire and burned area datasets. The current status highlights the validation protocols in place for reference data generation, product inter-comparisons, and the need for validation information for multiple use
0 views • 9 slides
Comparing Efficacy of LPV/r QD vs. BID in Combination Therapy
Study M02-418 compared once-daily (QD) and twice-daily (BID) LPV/r in combination with TDF and FTC for HIV treatment. The primary endpoint was achieving HIV RNA <50 c/mL at week 48, aiming for non-inferiority of QD vs. BID dosing. Patient characteristics, treatment response at week 48, and pharmacok
0 views • 7 slides
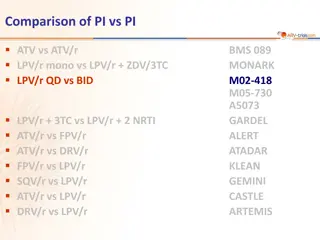
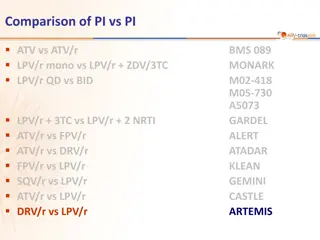
Comparison of Protease Inhibitors in ARTEMIS Study
This study compares the efficacy and safety of different protease inhibitors (PIs) including ATV, ATV/r, LPV/r, FPV/r, DRV/r, and SQV/r in HIV treatment. The ARTEMIS study specifically focuses on comparing DRV/r and LPV/r in combination with TDF/FTC in ARV-naive patients. Results show non-inferiorit
0 views • 12 slides