Enhancing Security Definitions for Functional Encryption
This study delves into the realm of functional encryption (FE) against probabilistic queries, highlighting the necessity for improved security definitions to address existing limitations such as counter-intuitive examples and impossibility results. The exploration leads to proposing a new security n
4 views • 20 slides
IVD Contract Manufacturing Services Market - Global Opportunity Analysis and Ind
IVD Contract Manufacturing Services Market by Type (Assay Development, Manufacturing), Category (Reagents, Systems), Technology (Immunoassay, Molecular Diagnostics, Clinical Chemistry, Hematology, Microbiology, Urinalysis) \u2013 Global Forecast to 2031\n
4 views • 4 slides
Comprehensive Guide to Precision Gear Manufacturing Processes
This comprehensive guide by Parkash Industrial Gears explores the manufacturing processes of worm gear manufacturing, bevel gears, spur gears, and helical gears. Learn about the precision crafting techniques and materials used to ensure durability and efficiency in these essential mechanical compone
0 views • 7 slides
Certification ISO
ISO \u2013 Organisation internationale de normalisation est une organisation ind\u00e9pendante qui d\u00e9finit des normes pour les entreprises en termes de qualit\u00e9, de s\u00e9curit\u00e9 et d\u2019environnement du syst\u00e8me de gestion. L\u20
2 views • 3 slides
Online Money by PTC Shares Through Affiliate Marketing
In the dynamic world of digital commerce, affiliate marketing stands as a pivotal force, driving sales and expanding market reach for businesses across diverse sectors. One sector that has notably embraced this strategy is the Paid-To-Click (PTC) ind
2 views • 7 slides
Understanding Regulatory Requirements of Drugs and Pharmaceuticals
Drug regulation involves controlling drug use through international agreement authorities like the FDA, EMA, and PMDA. The FDA plays a crucial role in drug evaluation and research, biologic evaluation, devices, and food safety. There are various types of applications for drug approval, along with a
0 views • 28 slides
Understanding Investigational New Drug Applications (INDA)
An Investigational New Drug Application (INDA) is a crucial submission to the FDA for permission to conduct clinical studies on new drug products. It plays a pivotal role in assessing the safety and efficacy of new drugs before they can be marketed and distributed for human use. This article covers
0 views • 30 slides
Operating Segments Identification and Reporting under IND AS 108
This article by Harshad B. Bhudia delves into the detailed concepts of IND AS 108 focusing on identifying the Chief Officer Decision Maker, aggregating segments, and determining reportable segments based on revenue criteria. The article provides a peaceful explanation of operating segments and the e
0 views • 15 slides
Employee Stock Option Plan Presentation and Guide
This presentation and guide cover the Employee Stock Option Plan, including Black-Scholes Model, practical challenges, market research, and relevant standards like Ind AS 102. It provides an in-depth overview of share-based payments, recognition, measurement, and disclosure requirements, along with
0 views • 71 slides
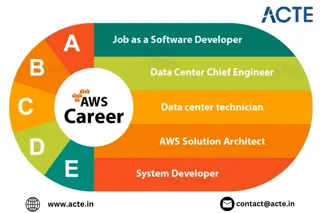
Exploring the Booming Job Opportunities for Amazon Web Services (AWS) Profession
Amazon Web Services (AWS) has revolutionized the cloud computing landscape on a global scale, and India is no exception. The demand for AWS skills in India is robust and continues to grow, driven by the rapid digital transformation across various ind
1 views • 1 slides
INVESTMENT PROPERTIES
Explore key definitions, measurements, and disclosures related to Investment Property under Ind-AS and AS-1, with examples distinguishing between investment property and non-investment property. Learn about dual-purpose properties and how to differentiate them for accounting purposes.
0 views • 24 slides
Obtaining TPOXX Treatment for MPV Infection in Michigan (July 22, 2022)
The process for obtaining TPOXX for treating patients with MPV infection in Michigan as of July 22, 2022 involves indications for treatment, information for healthcare providers, and updated CDC IND protocol changes. The required documentation includes informed consent, patient intake form, FDA Form
0 views • 8 slides
Evolution of Proofs in Cryptography
Cryptography has evolved from classical proofs to interactive and probabilistically checkable proofs, enabling the development of applications like Non-Malleable and Chosen-Ciphertext Secure Encryption Schemes. Non-Malleability protects against active attacks like malleability and chosen-ciphertext
0 views • 29 slides
Oblivious RAM and Software Protection: An Overview
Oblivious RAM (ORAM) and software protection against piracy involve securing hardware and encrypted programs to prevent unauthorized access. With a focus on achieving security through encryption and indistinguishability, concepts like access patterns and data request sequences play a crucial role. T
0 views • 26 slides
Access to Investigational Therapies: Regulations and Criteria
This content discusses the access to investigational drugs outside of clinical trials, highlighting Treatment Use Criteria and Emergency Use situations. It covers Subpart E regulations, including the definition of life-threatening and severely-debilitating illnesses. The importance of safeguards lik
0 views • 10 slides
Quality Issues in Clinical Trial Materials: CMC Review by Dr. Dorota Matecka
Clinical trial materials undergo Chemistry, Manufacturing, and Controls (CMC) review to ensure pharmaceutical quality. This process includes assessing safety concerns, impurities, and specifications, along with other CMC considerations. Pharmaceutical quality encompasses the suitability, identity, s
0 views • 41 slides
Shari Ann Chinnis Indianapolis: A Trailblazer in Leadership and Philanthropy
\ud83d\ude80 Shari Ann Chinnis is leading Indy\u2019s future! From philanthropy to education, her leadership inspires us all. Discover her story! \ud83c\udf1f \nRead More: \/\/chicpeekfashion.com\/shari-ann-chinnis-indianapolis\n#ShariAnnChinnis #Ind
0 views • 4 slides
Understanding FDA Regulations in Clinical Research
FDA regulations in clinical research cover the protection of human subjects, IND/IDE requirements, risk determinations, application processes, and what falls under FDA regulation. It distinguishes between FDA-regulated activities and those not regulated, such as medical record reviews. The scope inc
0 views • 31 slides
BIKE Cryptosystem: Failure Analysis and Bit-Flipping Decoder
The BIKE cryptosystem is a code-based KEM in the NIST PQC standardization process, utilizing the Niederreiter variant of the McEliece Construction with a QC-MDPC code. It ensures security against IND-CPA, and efforts are made to further confirm or disconfirm its estimates for IND-CCA security requir
0 views • 14 slides
JCB JCB380, JS370 [T2 IND] EXCAVATOR Service Repair Manual Instant Download (SN from 2500652 onwards)
Please open the website below to get the complete manual\n\n\/\/
0 views • 25 slides
Understanding Clinical Trials: Phases, Types, and Definitions
Clinical trials play a crucial role in advancing medical research and treatment options. This comprehensive guide covers the basics of clinical trials, including their definition, phases, types, and key definitions like IND, IDE, NDA, and more. Discover how different phases of trials work, the vario
0 views • 16 slides
Overview of BIKE Key Exchange Protocol by Ray Perlner
The BIKE (Bit-Flipping Key Exchange) protocol, presented by Ray Perlner, offers variants based on MDPC codes like McEliece and Niederreiter with a focus on quasi-cyclic structures. These non-algebraic codes show promise for key reduction, targeting IND-CPA security. The protocol features competitive
0 views • 18 slides


















![JCB JCB380, JS370 [T2 IND] EXCAVATOR Service Repair Manual Instant Download (SN from 2500652 onwards)](/thumb/243417/jcb-jcb380-js370-t2-ind-excavator-service-repair-manual-instant-download-sn-from-2500652-onwards.jpg)

