Proposed Ratification of the 2003 Convention on Safeguarding Intangible Cultural Heritage
The briefing presents a proposal for the ratification of the 2003 UNESCO Convention on Safeguarding Intangible Cultural Heritage (ICH). The Convention aims to safeguard, preserve, and promote ICH, which includes practices, knowledge, skills, and cultural spaces passed down through generations. It em
0 views • 19 slides
Understanding the Relationship Between Intangible Cultural Heritage and Disaster Risk Reduction
This PowerPoint presentation by UNESCO explores the connection between Intangible Cultural Heritage (ICH) and Disaster Risk Reduction (DRR). It covers key concepts like disasters, risks, vulnerabilities, and resilience, emphasizing the importance of safeguarding and mobilizing ICH in the face of dis
7 views • 23 slides
4 bài tập thể dục cho bà bầu 3 tháng đầu giúp ích cho mẹ và bé
Vi\u1ec7c m\u1eb9 b\u1ea7u t\u1eadp luy\u1ec7n c\u00e1c b\u00e0i t\u1eadp th\u1ec3 d\u1ee5c cho b\u00e0 b\u1ea7u 3 th\u00e1ng \u0111\u1ea7u \u0111\u1ec1u \u0111\u1eb7n, th\u01b0\u1eddng xuy\u00ean s\u1ebd gi\u00fap t\u1ea1o d\u1ef1ng m\u1ed9t n\u1ec1n t\u1ea3ng s\u1ee9c kh\u1ecfe t\u1ed1t nh\u1ea5t
2 views • 3 slides
Implementation of Estimand Framework in U.S. Regulatory Landscape
The regulatory implementation of the Estimand Framework in the U.S. context has evolved over time, addressing issues related to missing data in clinical trials. Starting from the pre-history of missing data recognition to the development of the ICH E9(R1) framework, the focus has been on aligning pl
0 views • 15 slides
Understanding Estimands in Oncology Clinical Trials
The concept of estimands in oncology clinical trials plays a crucial role in defining treatment effects and analyzing overall survival outcomes. This framework aims to enhance transparency, align trial objectives, and strengthen interdisciplinary dialogues. Introduced by the ICH E9(R1) guideline in
0 views • 14 slides
Ensuring Protocol Compliance and Corrective Action Plans in Clinical Trials
This content discusses the importance of creating Corrective Action and Preventive Action (CAPA) plans for protocol deviations in clinical trials. It covers the components of a CAPA, best practices for creating CAPAs for different deviation types, and regulatory compliance requirements according to
0 views • 33 slides
Understanding Essential Documents in Clinical Trials
This presentation by Derita Bran, BSN, RN, CCRC, focuses on the documentation required for quality clinical trials, emphasizing Essential Documents outlined in the ICH GCP E6 (R2) guidance. It covers the purpose, definition, and importance of these documents in ensuring subject rights protection, da
4 views • 35 slides
Effective Protocol Deviation Management in Clinical Research
Understand the importance of handling protocol deviations in clinical trials to ensure patient safety, data integrity, and compliance with regulatory requirements. Explore SOPs, electronic systems, and best practices for managing deviations effectively. Learn about GCP requirements, DMC usefulness,
0 views • 22 slides
Understanding Investigational Products in Clinical Trials
Investigational products play a crucial role in clinical trials, encompassing drugs, devices, biologics, and more. The FDA defines investigational new drugs as substances seeking approval, even if previously in use, with potential changes. Similarly, investigational devices are those under investiga
0 views • 43 slides
Understanding Informed Consent in Research
Explore the fundamentals of informed consent in research, including ICH G6 (R2) guidance, study resources, webinars, and contact information for further inquiries at dbran@uthsc.edu and mlynn@uthsc.edu.
0 views • 76 slides
International Conference on Harmonization in Pharmaceutical Registrations
The International Conference on Harmonization (ICH) aims to harmonize technical requirements for pharmaceutical registrations globally. Through the Common Technical Document (CTD) framework, it promotes efficiency, cost-effectiveness, and high quality in medicine development. The ICH provides recomm
0 views • 29 slides
Canine Infectious Hepatitis (ICH) - Overview, Symptoms, and Treatment
Canine Infectious Hepatitis (ICH), also known as Rubarth's Disease, is caused by Canine Adenovirus-1 (CAdV-1), affecting dogs of all ages. The virus primarily targets hepatocytes, leading to acute hepatitis, as well as respiratory and ocular issues. Transmission occurs through contact with infected
6 views • 7 slides
Lợi ích khi mẹ bầu cho thai nhi nghe nhạc
Trong qu\u00e1 tr\u00ecnh mang thai, c\u00f3 r\u1ea5t nhi\u1ec1u ph\u01b0\u01a1ng ph\u00e1p c\u00f3 th\u1ec3 \u00e1p d\u1ee5ng nh\u1eb1m th\u00fac \u0111\u1ea9y s\u1ef1 ph\u00e1t tri\u1ec3n c\u1ee7a con. Trong \u0111\u00f3, s\u1eed d\u1ee5ng \u00e2m
0 views • 3 slides
Understanding Estimands at ICH for Improved Trial Objectives
Lack of clarity in trial objectives can lead to confusion among developers, decision-makers, patients, and prescribers. Estimands at ICH, presented by Rob Hemmings, address the need to focus on WHAT to estimate rather than just HOW. Patients differ in response to treatment, and multiple treatment ef
0 views • 13 slides
Integrating Disaster Risk Reduction into ICH Inventorying for UNESCO
Learn how to apply a community-based approach to inventorying and safeguarding intangible cultural heritage within the context of disasters. This presentation offers frameworks, tools, and exercises to integrate disaster awareness into ICH inventorying and safeguarding practices, emphasizing the imp
0 views • 15 slides
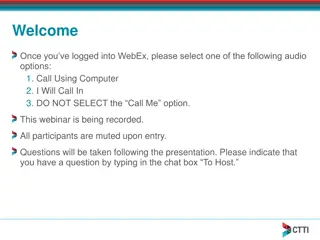
Enhancing Clinical Trials through ICH E6 Renovation
Explore the Clinical Trials Transformation Initiative's efforts in updating ICH E6 guidelines to improve global clinical trials. Learn about CTTI's findings, insights from stakeholders, and discussions on the necessary updates for ICH E6. Engage with industry experts and regulatory authorities to un
0 views • 65 slides
Insights from CTTI ICH E6 Survey and Stakeholder Input
Explore key findings from the Clinical Trials Transformation Initiative (CTTI) ICH E6 Survey, identifying areas in need of updating and stakeholder perspectives. Survey participants, from various regions, emphasized updating top ICH E6 principles like quality assurance systems and informed consent.
0 views • 21 slides
Active Ageing Campaign in Public Areas - Promoting Health and Well-being
The campaign "Ich bewege mich, mir geht es gut" aims to promote active ageing by encouraging physical activity in public areas. Initiated by LZG Landeszentrale für Gesundheitsförderung and supported by the Rheinhessischer Turnerbund, the campaign focuses on engaging elderly individuals in various
0 views • 20 slides
Utilizing Bayesian Hierarchical Model for Clinical Trial Quality Design
Explore how a Bayesian Hierarchical Model can be leveraged to design quality into clinical trials and ensure compliance with ICH E6 R2 Quality Tolerance Limits. Learn about the Risk-Based approach, Quality Tolerance Limits methodology, and the application of Bayesian modeling for early phase studies
0 views • 14 slides
Một Số Lợi Ích Khi Uống Nước Mía Cho Bà Bầu
Theo c\u00e1c chuy\u00ean gia n\u01b0\u1edbc m\u00eda c\u00f3 th\u1ec3 cung c\u1ea5p nhi\u1ec1u vitamin v\u00e0 kho\u00e1ng ch\u1ea5t c\u00f3 l\u1ee3i cho s\u1ee9c kh\u1ecfe thai k\u1ef3. C\u1ee5 th\u1ec3 b\u00e0 b\u1ea7u u\u1ed1ng n\u01b0\u1edbc m\u
0 views • 3 slides