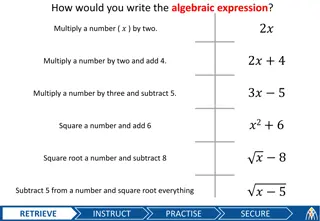
Mastering Algebraic Expressions and Formula Rearrangement
Learn to write algebraic expressions, manipulate formulas, calculate costs based on equations, and change formula subjects efficiently.
0 views • 14 slides
Favorskii Rearrangement in Organic Chemistry
Favorskii rearrangement is a base-catalyzed rearrangement reaction of halo ketones giving rise to acid, ester, or amine compounds via a cyclopropane intermediate. The mechanism, evidence supporting it, variations in reaction based on the presence of hydrogen, and stereospecificity are discussed with
0 views • 8 slides
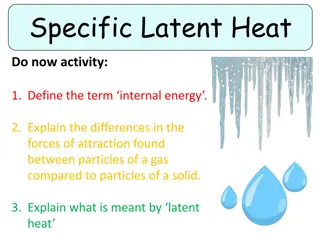
Specific Latent Heat and Particle Changes
Internal energy, forces of attraction in gases vs. solids, and latent heat concepts are explained. Particles changing state are visualized through a graph. Self-assessment points and the calculation for specific latent heat of fusion are discussed. The rearrangement of the equation for specific late
2 views • 20 slides
Rearrangements in CBCS Semester IV System - Reactions and Mechanisms
Dr. Mumu Chakraborty, Assistant Professor at Government Girls General Degree College, presents detailed lectures on rearrangements in the CBCS Semester IV system. Topics covered include diazonium salts, electron-deficient carbon rearrangements, nitrogen and oxygen rearrangements, and aromatic rearra
1 views • 25 slides
Dakin Rearrangement in Organic Chemistry: Mechanism and Positional Effects
The Dakin Rearrangement, also known as Dakin oxidation, is an organic redox reaction involving hydroxylated phenyl aldehydes or ketones reacting with hydrogen peroxide to form benzenediols and carboxylates. The mechanism includes nucleophilic addition, [1,2]-aryl migration, and final product formati
2 views • 9 slides
Overview of Organic Chemistry Rearrangement Reactions
Explore different rearrangement reactions in organic chemistry such as Curtius rearrangement, Lossen rearrangement, and Schmidt rearrangement. Understand the mechanisms, applications, and examples of these reactions in synthesizing various compounds like isocyanates, amines, and urea derivatives.
0 views • 19 slides
Tablet Compression and Compaction in Modern Pharmaceutics
This educational material delves into the physics of tablet making, focusing on the forces involved, compaction profiles, and effects of friction. Students will gain knowledge about tablet compression, compaction, and consolidation, including the types of forces at play and the distribution of force
2 views • 16 slides
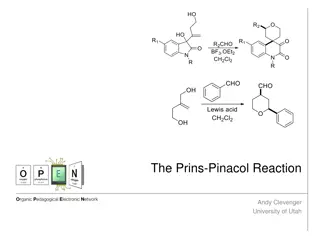
The Prins-Pinacol Reaction in Organic Chemistry
The Prins-Pinacol reaction involves a two-step process starting with the Prins reaction and followed by the Pinacol rearrangement. This reaction, discovered in 1919 by Hendrick J. Prins, is a crucial transformation in organic chemistry, leading to the formation of important carbonyl compounds. The m
0 views • 14 slides
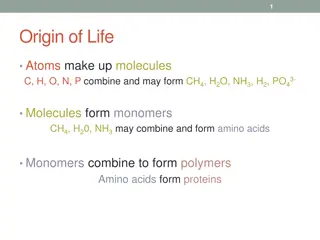
Chemical Reactions and Energy Transfer in Biology
Exploring the origin of life, the formation of molecules, and the transition to proteins through amino acids. Definitions of key terms in biology, the role of enzymes, and the significance of chemical reactions in rearranging atoms and transferring energy are discussed. Various forms of energy and t
0 views • 32 slides
Proteomics Data Analysis Workflows in Perseus
This content provides a detailed walkthrough of utilizing Perseus interface/functions for analyzing label-free and SILAC datasets in the field of proteomics. It covers loading, filtering, visualization, log transformation, rearrangement of columns, and advanced analysis techniques such as scatter pl
2 views • 4 slides
Benzidine Rearrangement: Mechanism and Substituent Effects
Benzidine rearrangement involves the conversion of hydrazobenzenes into a mixture of benzidine and diphenylene under acidic conditions. Various substituents influence the product formation, with o-/m- positions favoring benzidines, and p- positions leading to different products based on the nature o
0 views • 15 slides
Chemical Reactions and Reactivity Series
Chemical reactions involve the rearrangement of atoms, with reactants forming products. Different signs indicate a chemical reaction, such as gas release, odor, energy change, color change, and solid formation. Equations model these changes, showing the conservation of mass. Reactivity series help u
0 views • 6 slides
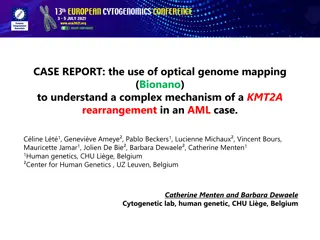
Complex KMT2A Rearrangement in AML with Optical Genome Mapping
A case report explores the use of optical genome mapping (OGM) to unravel a complex KMT2A rearrangement in an AML patient. Traditional cytogenetic analyses identified translocations and rearrangements involving chromosomes 10 and 11, leading to the fusion of genes KMT2A and MLLT10. OGM technique, ut
3 views • 5 slides
Hofmann Rearrangement: Mechanism, Stereochemistry, and Key Steps
The Hofmann rearrangement is a notable organic chemistry reaction that converts an amide into an amine with one less carbon atom. This process involves key steps such as bromination of nitrogen, extraction of H+ by OH-, and rearrangement of anion. The mechanism includes the formation of N-Bromoamide
6 views • 15 slides
Aromatic Rearrangement Mechanisms in Organic Chemistry
Aromatic rearrangements are key transformations in organic chemistry involving intramolecular and intermolecular rearrangements of aromatic systems. Examples include Fischer-Hepp rearrangement, Hofmann-Martius rearrangement, N-Azo to C-Azo rearrangement, Bamberger rearrangement, and Orton rearrangem
0 views • 11 slides
Road Safety Audit Seminar Update - May 2018
Latest statistics and updates from the Road Safety Audit Seminar held in May 2018, covering a comprehensive overview of 17 years of road safety audit data, including the number of auditors registered, team leaders, active auditors, completed audits from 2010-2017, and updates on the latest standards
1 views • 26 slides
The Pinacol-Pinacolone Rearrangement in Organic Chemistry
The Pinacol-Pinacolone rearrangement is a crucial process in organic chemistry for converting 1,2-diols into carbonyl compounds. This rearrangement involves a 1,2-migration under acyl conditions, resulting in the shift of two adjacent atoms. Learn about the mechanisms, products, and uses of Pinacolo
0 views • 11 slides
Python Lists Operations
Python lists are versatile data structures that allow for efficient creation, querying, modification, insertion, removal, replacement, and rearrangement of elements. This comprehensive guide covers essential list operations such as extracting parts of a list, finding elements, and altering list cont
0 views • 18 slides
Comparison between Array and Linked List Data Structures
Linked lists and arrays are commonly used data structures in programming. Linked lists offer flexibility in size changes and efficient rearrangement of elements, while arrays provide direct access to elements based on their index. Linked lists involve pointers connecting elements, allowing for dynam
0 views • 24 slides
The Synthesis of Cedranoid Sesquiterpenes via Photo-Rearrangement of Bicyclo[2.2.2] Octenones
Peter Yates, a renowned chemist, published over 200 papers and contributed significantly to the field of chemistry. His work on the structural characterization of various compounds, including Mangosteen and Shellolic Acid, is well-recognized. This article focuses on the synthesis of Cedranoid Sesqui
0 views • 10 slides
Optimizing ROS1 Targeted Therapies in NSCLC
Lung cancer, particularly Non-Small Cell Lung Cancer (NSCLC), poses significant challenges in diagnosis and treatment. This content delves into the intricacies of ROS1 rearrangement in NSCLC, exploring current treatment options, optimizing targeted therapies, and assessing emerging treatment modalit
0 views • 19 slides
Efficiency Equation Rearrangement & Calculation Examples
Learn how to rearrange and calculate efficiency using the efficiency equation in machines. Understand the concept of efficiency, its limits, related equations, and work output calculations with step-by-step examples.
0 views • 10 slides
Comprehensive Analysis of Cancer Genes Deregulated by Genomic Rearrangements
Investigation of somatic structural variation effects on gene expression in 1220 cancers reveals upregulation of key cancer genes such as TERT, MDM2, CDK4, and ERBB2. The study delves into the relationship between rearrangement-mediated cis-regulatory alterations and gene deregulation using a high-c
0 views • 21 slides
Is Baptism for the Remission of Sins?
In Acts 2:36-39 and Mark 16:16, the significance of baptism for the remission of sins is explored. The verses highlight the importance of belief, baptism, and salvation. The discussion touches on differing perspectives among Baptists and Catholics regarding the role of baptism in salvation. Addition
0 views • 20 slides













![The Synthesis of Cedranoid Sesquiterpenes via Photo-Rearrangement of Bicyclo[2.2.2] Octenones](/thumb/198279/the-synthesis-of-cedranoid-sesquiterpenes-via-photo-rearrangement-of-bicyclo-2-2-2-octenones.jpg)



