Overview of UW Clinical Trial Office Budget Review
UW Clinical Trial Office conducts budget reviews to ensure compliance and financial accountability in clinical trials. The office collaborates with various departments to manage billing compliance, financial risks, and institutional policies. The primary focus is on avoiding patient billing errors,
0 views • 17 slides
India Alliance Clinical & Public Health fellowship in India
India Alliance Clinical & Public Health fellowship in India\n\nIndia Alliance Clinical and Public Health Research Fellowships are for Health researchers with an MD, MS, MPH, or an equivalent clinical or public health degree, who can apply for the DBT\/Wellcome Trust India Alliance Clinical and Publi
0 views • 5 slides
Clinical Escalation: Building Effective Communication in Maternity Units
Exploring the importance of clinical escalation in maternity units, this session outlines the components and practices involved in identifying, communicating, and acting upon clinical concerns. It emphasizes recognizing deviation from normality, effective communication, and taking appropriate action
0 views • 27 slides
Training Models in Clinical Psychology: A Comprehensive Overview
Clinical psychology training models such as the Scientist-Practitioner Model have evolved over the years to integrate science and practical expertise. The Scientist-Practitioner Model, originating in 1949, emphasizes the fusion of scientific knowledge and clinical application. It faced criticism for
1 views • 98 slides
RRH Student Re-Entry Process for Clinical Requests & Compliance
The RRH Student Re-Entry Process outlines steps for clinical request processing, approval, and compliance requirements for various disciplines within the nursing program and other departments. It includes sending requests to the Nursing Institute, communication procedures, compliance documentation,
0 views • 10 slides
Ethical Issues in Clinical Pharmacy Research by Dr. Haider Raheem Mohammad
Research ethics play a crucial role in clinical trials and therapeutic research in the field of pharmacy. From discovery to validation, all medicines undergo rigorous evaluation processes to ensure safety, efficacy, and freedom from adverse effects. Clinical trials in both animals and humans are ess
0 views • 20 slides
Advanced Clinical Practice Framework and Pillars of Practice
The document discusses the advanced clinical practice framework and the four pillars of practice which include leadership & management, clinical practice, education, and research. It emphasizes the importance of core capabilities and area-specific competence in advanced clinical practice. The role o
2 views • 8 slides
Digital Health Technology-Derived Clinical Outcome Assessments in Regulatory Decision-Making
This session discusses the landscape of DHT-derived novel endpoints in clinical research, focusing on considerations for regulatory decision-making. It explains the use of digital health technology in clinical outcome assessments and highlights the potential benefits of digitally collected COAs, suc
0 views • 42 slides
Objective Structured Clinical Examination (OSCE): A Modern Approach to Assessing Clinical Competence
The Objective Structured Clinical Examination (OSCE) is a modern examination method widely used in the field of health science to evaluate clinical skill performance. It involves stations where medical students interact with simulated patients to demonstrate competencies such as history taking, phys
1 views • 40 slides
NIMH Clinical Research Education and Monitoring Program Overview
NIMH's Clinical Monitoring and Clinical Research Education, Support, and Training Program (CREST) aims to ensure the proper conduct, recording, and reporting of clinical trials. This program includes clinical monitoring plans, guidelines for site monitoring activities, and independent clinical monit
1 views • 29 slides
Understanding Statistical Methods for Clinical Endpoints in Diabetes Research
This educational slide module delves into fundamental statistics for analyzing clinical endpoints in diabetes research. It covers the choice of statistical methods, the distinction between statistical and clinical significance, and the importance of different endpoints in evaluating clinical benefit
1 views • 37 slides
Understanding Ethics in Clinical Trials: A Comprehensive Overview
Explore the historical context, important ethical guidelines, and the ethical framework with 7 principles in the field of clinical trials. Learn about key trials, ethical considerations, and guidelines governing human subject research in clinical medicine. Delve into the critical aspects such as inf
1 views • 15 slides
Understanding Non-Clinical Development in Therapeutic Innovation
The European Patients Academy on Therapeutic Innovation focuses on the non-clinical development phase of medicine, delving into efficacy assessment, safety evaluation, and manufacturing process considerations. Non-clinical studies are essential for decision-making in clinical trials, marketing appli
1 views • 26 slides
UVA Clinical Nursing Preceptor Training Regulations
Virginia Board of Nursing regulations outline the role of clinical preceptors in supervising nursing students, ensuring safe client care, and enhancing clinical learning experiences. Preceptors serve as teachers, mentors, and role models, linking classroom knowledge with practical skills. Faculty me
0 views • 36 slides
Understanding Essential Documents in Clinical Trials
This presentation by Derita Bran, BSN, RN, CCRC, focuses on the documentation required for quality clinical trials, emphasizing Essential Documents outlined in the ICH GCP E6 (R2) guidance. It covers the purpose, definition, and importance of these documents in ensuring subject rights protection, da
3 views • 35 slides
Importance of Communication and Counseling Skills in Clinical Settings
Dr. Leena Baghdadi, an Assistant Professor and Clinical Epidemiologist, emphasizes the significance of effective communication and counseling skills in clinical practice. The content discusses the objectives of understanding these skills, barriers, and practical examples of counseling. Highlighted b
0 views • 27 slides
BMC Clinical Trials Office Goals and Initiatives
The BMC Clinical Trials Office (CTO) is dedicated to supporting clinical and human research at Boston Medical Center by providing leadership and expertise in research, finance, and administration. The CTO reviews, negotiates, and approves protocols, supports grants and contracts, ensures accurate cl
0 views • 17 slides
Understanding Clinical Trials: Types and Designs
Clinical trials are essential research studies that evaluate new tests and treatments to improve human health outcomes. They involve various phases, designs, and purposes, such as treatment trials, prevention trials, and observational studies. Different types of clinical trial designs include experi
7 views • 18 slides
Veterinary Medicinal Products Clinical Trials in Ireland
This presentation covers the process and regulations surrounding clinical trials and testing of veterinary medicinal products in Ireland. Topics include the application process, HPRA evaluation, fees, scope of legislation, and the purpose of clinical trials. Feedback from a 2016 HPRA survey on clini
3 views • 17 slides
Understanding Clinical Validation and DRG Validation in Healthcare
Clinical validation ensures that diagnoses documented in a patient's record align with accepted clinical criteria, while DRG validation focuses on matching hospital-coded information with physician descriptions and patient records. Clinical validation involves a review by clinicians to confirm the p
1 views • 39 slides
Dr. Chris Webb's December 2020 Clinical Supervisors Report
A structured Clinical Supervisors Report (CSR) for Dr. Chris Webb in December 2020. This report provides insight into the clinical supervision process within various posts and includes valuable information from the Clinical Supervisor.
0 views • 26 slides
Enhancing Compliance Monitoring in South African Public Service
The Compliance Monitoring Framework developed by the Office of Standards and Compliance aims to improve adherence to public administration norms and standards in South Africa. This framework is designed to reduce non-compliance through ongoing supervision, investigation, and promotion of proper beha
3 views • 19 slides
Effective Corporate Compliance Program Overview
Corporate compliance is essential to prevent fraud, waste, and abuse within organizations. This program aims to detect and prevent deceptive practices, unnecessary costs, and improper behaviors. Key elements include appointing a Compliance Officer, establishing policies and procedures, providing edu
1 views • 19 slides
Understanding Evidence-Based Medicine and Clinical Decision-Making
European Patients Academy on Therapeutic Innovation emphasizes the importance of Evidence-Based Medicine (EBM) in providing optimum clinical care. EBM involves systematic review and utilization of clinical research for informed decision-making, benefiting patients in disease management and treatment
7 views • 20 slides
Navigating Challenges in Clinical Rotations for Health Professions Faculty
This presentation highlights the challenges and choices faced by health professions faculty in clinical rotations, focusing on identifying opportunities and resources for limited clinical experiences. The content covers fundamental terminology, options available, and strategies such as on-site and r
1 views • 18 slides
Post Award Fiscal Compliance: Who We Are and What We Do
Post Award Fiscal Compliance (PAFC) assists campus and central administrative units in mitigating non-compliance risks with sponsor terms and conditions by monitoring compliance, interpreting award requirements, providing training, and enhancing internal controls. The team includes Matt Gardner, Ass
0 views • 11 slides
Essential Aspects of the Clinical Interview in Psychology
Clinical interviews play a crucial role in the assessment conducted by clinical psychologists, showcasing essential qualities like validity, reliability, and clinical utility. Understanding the importance of feedback and honing general and specific skills as an interviewer are key components in cond
1 views • 17 slides
Comprehensive Guidance for Clinical Staff on Complaints Handling Process
Developed for clinical professionals, this guidance provides support for participating in complaints investigations and writing clinical reports. It aims to clarify the definition of clinical judgment and outlines the key elements of handling complaints effectively. The module encourages collaborati
0 views • 19 slides
Deanery of Clinical Sciences Funding Challenge 2018 Launch Event
The Deanery of Clinical Sciences is offering a small grant opportunity for early career researchers within the clinical sciences field. The fund aims to support researchers' current studies and research projects with a maximum grant of £2,500. Applications are open to postgraduates and postdocs, an
3 views • 7 slides
Compliance Requirements for Athletic Training Graduate Program
This program outlines the compliance requirements for the Athletic Training Graduate Program, including background checks, drug screenings, and clinical requirements such as immunizations and certifications. Important due dates and instructions on how to get started are provided to ensure timely com
0 views • 25 slides
Clinical Research Guidelines and Regulations Overview
Clinical research encompasses various guidelines and regulations to ensure the protection of human subjects and the credibility of study results. Key aspects include Good Clinical Practice (GCP) standards, Title 45 of the Code of Federal Regulations (CFR) Part 46, and additional CFR sections for cli
0 views • 46 slides
Ohio Clinical Alliance: Transforming Clinical Experiences
The Ohio Clinical Alliance, through collaborative partnerships, aims to enhance clinical preparation for educators. The leadership team comprises various representatives and organizations committed to improving student learning. Their activities include retreats and meetings to ensure effective comm
0 views • 27 slides
Importance of Compliance Training in Ensuring Ethical Business Practices
Compliance training at West Cancer Center plays a vital role in educating employees on laws, regulations, and company policies to ensure ethical conduct. With a commitment to compliance, the center's Code of Ethics emphasizes activities that adhere to laws and regulations, prioritize quality care, a
0 views • 31 slides
Perception and Awareness of Clinical Research in Trial Participants and the Public of Andhra Pradesh
This study focuses on understanding the perception and awareness of clinical research among trial participants and the general public in Andhra Pradesh. It highlights the importance of creating awareness about clinical research, previous study results, public attitudes towards clinical trials, and e
0 views • 24 slides
Statistical Issues in Clinical Trials: Insights from 13th Annual Conference
The 13th annual conference on Statistical Issues in Clinical Trials covered topics such as penalties for extra variation and limited degrees of freedom, the Diet-Heart Hypothesis, controlled trials, unit of randomization, and causal inference. Speakers highlighted the importance of addressing cluste
0 views • 10 slides
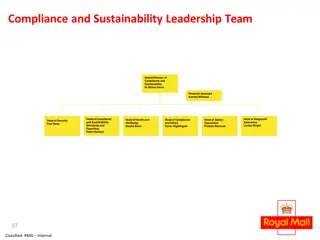
Compliance and Sustainability Leadership Team Overview
This document provides an overview of the Compliance and Sustainability Leadership Team structure, including key positions such as Global Director, Head of Compliance and Sustainability Standards, and various advisors and managers responsible for safety, health, compliance, ethics, and risk manageme
0 views • 6 slides
Compliance Assurance Report on Dental Services in Q1 2024
A compliance assurance report was conducted on dental services in Q1 2024 as part of the HSE Children First Compliance Assurance Checks. The report highlighted areas of compliance and partial compliance, with efforts noted to meet Children First requirements. Areas of improvement were identified in
0 views • 19 slides
Comparison of Professional Behaviors in Clinical Education
Professional behavior characteristics play a crucial role in enhancing student learning during clinical education. This study examines the differences in reported importance and frequency of professional behaviors between credentialed and non-credentialed clinical instructors. The background outline
0 views • 28 slides
Compliance Testing by ERA for IT Systems Developed and Deployed for TAF TSI
Compliance testing for IT systems developed and deployed for TAF TSI involves checking if messages comply with TAF XSD and basic parameters, assessing compliance of IT tools against TSI requirements, and issuing compliance assessment reports. The process includes testing messages for mandatory eleme
0 views • 4 slides
Addressing Compliance Challenges in Ottawa's Housing Sector
Ottawa's housing organizations face compliance challenges related to fire safety, including lack of code adherence, non-compliance issues, and legacy structural issues. Efforts to achieve compliance involve daily inspections, addressing combustible materials and obstructions, and obtaining funds for
0 views • 11 slides