Understanding Electrons in Atoms: Models and Quantum Mechanics
Explore the evolution of atom models from Rutherford to Bohr and the Quantum Mechanical Model. Learn about energy levels, electron movement, excitations, and orbital probabilities. Discover how electrons jump between energy levels and the limitations of Bohr's model in multi-electron atoms.
1 views • 51 slides
Exploring Quantum Theory and the Atom: Electrons in Atoms and the Periodic Table
Delve into the fascinating world of quantum theory and the atom in Chapter 9, where we compare Bohr's model with the quantum mechanical model. Understand de Broglie's wave-particle duality and Heisenberg's uncertainty principle's impact on our current electron view. Discover the relationships among
0 views • 31 slides
Discovering Denmark, Sweden, and Finland: Member Countries of the EU
Denmark, Sweden, and Finland are vibrant member countries of the European Union with rich historical sites, cultural specialties, and notable personalities. Each country boasts unique attractions like Frederiksborg Castle in Denmark, Drottningholm Palace in Sweden, and Suomenlinna Fortress in Finlan
0 views • 4 slides
Understanding Atomic Structure: Electrons, Energy Levels, and Historical Models
The atomic model describes how electrons occupy energy levels or shells in an atom. These energy levels have specific capacities for electrons. The electronic structure of an atom is represented by numbers indicating electron distribution. Over time, scientists have developed atomic models based on
0 views • 5 slides
Understanding Atoms and Molecules: Basics and Models
Explore the world of atoms and molecules with a focus on distinguishing between them, understanding atomic structures through historic models like Bohr and Rutherford, and the modern atomic model. Discover how atoms form the basis of all matter and how they are organized within molecules.
0 views • 20 slides
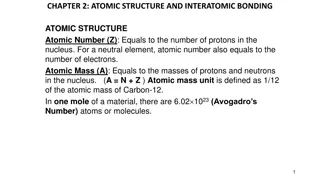
Understanding Atomic Structure and Interatomic Bonding
Atomic structure is defined by the atomic number (Z) and atomic mass (A). Quantum mechanics governs atomic and subatomic particles, introducing discrete energy levels. The Bohr atomic model describes electrons orbiting the nucleus in defined orbitals. Quantum numbers characterize electron properties
0 views • 15 slides
Exploring De Broglie Waves, Bohr's Quantization, and Electron Scattering in Physics
Discover the fascinating concepts of De Broglie waves, Bohr's quantization conditions, and electron scattering in physics. Delve into the wave-particle duality, electron double-slit experiments, and the groundbreaking observations by Davisson and Germer. Uncover the implications of mass particles ha
0 views • 23 slides
Evolution of Atomic Models: From Thomson to Bohr
Scientists from J.J. Thomson to Niels Bohr made groundbreaking discoveries in understanding the structure of the atom. Thomson's plum-pudding model was followed by Rutherford's nuclear model, revealing the nucleus. Bohr introduced the concept of discrete energy levels and orbits for electrons. These
4 views • 22 slides
Nuclear Shapes at Critical Point of U(5)-SU(3) Phase Transition
Exploring nuclear shapes at the critical point of the U(5)-SU(3) nuclear shape phase transition using Bohr Hamiltonian with a sextic oscillator potential. The study investigates the transition from a spherical vibrator (U(5)) to a prolate rotor (SU(3)), providing insights into the Interacting Boson
0 views • 10 slides
Understanding Valence Electrons and Lewis Dot Diagrams
Explore the concept of valence electrons and Lewis dot diagrams in chemistry. Learn how to identify the number of protons, neutrons, and electrons in an element using Bohr model drawings. Discover the significance of valence electrons in bonding and how to determine the number of valence electrons f
0 views • 37 slides
Understanding Atomic Structure: The Journey from Ancient Philosophers to Modern Theories
Exploring the evolution of atomic theory from ancient times to John Dalton's contributions, the concept of indivisible atoms forming elements, atomic spectra of hydrogen, and Bohr's atomic model. Delve into the limitations of Bohr's model and the complexities of multi-electron atoms.
0 views • 15 slides
Evolution of Atomic Models: From Ancient Philosophers to Quantum Mechanics
Tracing the evolution of atomic models from the ancient Greek philosophers' concept of indivisible atoms to the groundbreaking discoveries of electrons, protons, and neutrons. The journey through Thomson's Plum Pudding model, Rutherford's planetary model, Bohr's quantized model, and the introduction
0 views • 14 slides
Understanding Atoms: From Structure to Models
Explore the fundamental building blocks of matter - atoms. Discover what materials are made of, delve into atomic theories, examine Bohr models, and learn about the intricate components such as protons, neutrons, and electrons. Engage in a journey through the microscopic world of atoms and their sig
0 views • 34 slides
Evolution of Atomic Models: From Dalton to Bohr
Explore the progression of atomic models from John Dalton's theory of tiny, structureless particles to J.J. Thomson's Plum Pudding model, Ernest Rutherford's discovery of the nucleus, and finally Niels Bohr's model with fixed electron orbits and energy levels. Witness the journey of scientific under
0 views • 28 slides
Evolution of Atomic Models: From Rutherford to Quantum Mechanics
Various atomic models have been proposed throughout history, starting with John Dalton's idea of atoms as tiny particles to J.J. Thomson's Plum Pudding model. Ernest Rutherford's discovery of the nucleus and Niels Bohr's quantum model of the atom revolutionized our understanding. Bohr's proposal of
0 views • 38 slides
Development of the Atomic Model: From Dalton to Rutherford
Before the discovery of the electron, John Dalton proposed the solid sphere model for elements, while JJ Thomson's experiments led to the discovery of electrons. Ernest Rutherford's alpha particle scattering experiment revealed the concentrated mass at the nucleus, which formed the basis of the nucl
0 views • 4 slides
Quantum Chemistry Learning Goals and Concepts
This content covers the learning goals and concepts of quantum chemistry leading up to the Schrodinger equation and potential energy wells, excluding the material on the hydrogen atom introduced later. It explores models of the atom, including observations of atomic spectra, the Bohr model, de Brogl
0 views • 22 slides
Quantum Theory and Key Figures in Physics
Explore the evolution of quantum theory through the perspectives of renowned physicists such as Albert Einstein, Niels Bohr, Werner Heisenberg, Erwin Schrödinger, Prince Louis de Broglie, and Max Planck. Learn about atomic line spectra, fundamental equations, and models used to represent the atom.
0 views • 23 slides
Understanding the Basics of Atom Structure in Electronics
Learn about the fundamental concepts of atom structure in electronics, including the components of atoms, the Bohr model, electron orbits, energy levels, valence electrons, and more. Understanding these principles is essential for grasping the underlying mechanisms of electronic devices.
0 views • 18 slides
Understanding Bohr's Model of the Hydrogen Atom
Exploring the significance of Bohr's hydrogen model in physics, this lecture delves into the Bohr radius, the correspondence principle, and the success and limitations of his model. Discover how characteristic X-ray spectra contribute to our understanding of atomic structures, leading to the conclus
0 views • 14 slides
Unveiling the Significance of the EPR Paradox and Bell's Inequalities
Physicists Albert Einstein, Boris Podolsky, and Nathan Rosen introduced the EPR paradox in 1935 to challenge the completeness of quantum mechanics through a thought experiment, highlighting issues in quantum entanglement and observable properties. Bohr's reaction emphasized the incompatibility of EP
0 views • 9 slides
Exploring Properties of Light and Models of the Atom in Chemistry
Delve into the fascinating world of light properties and atom models in chemistry. Unravel the scientific process, from successes to flaws, and master concepts like wavelength, frequency, and amplitude. Explore key experiments and models such as the Rutherford, Bohr, and DeBroglie models, as well as
0 views • 24 slides