Understanding Water, Acids, and Bases in Chemistry
Water is a vital substance on Earth, with its unique structure and properties making it essential for life. This chapter delves into the distribution of Earth's water, the chemical composition of water, the concepts of acids, bases, and pH, and how water's properties are influenced by hydrogen bondi
1 views • 8 slides
Understanding Acid-Base Balance in the Body: Importance and Regulation
Acid-base balance is crucial for maintaining optimal health, as slight deviations in hydrogen ion concentration can impact enzyme activity and metabolic processes. The body employs various defense mechanisms to regulate pH levels, involving buffers, lungs, and kidneys. Strong acids release more H+ i
3 views • 48 slides
Understanding Acids, Bases, and Buffers in Medical Biochemistry
Biologically important molecules, such as acids and bases, have significant roles in metabolism. Strong acids like hydrochloric acid ionize completely, while weak acids and bases play crucial regulatory roles. The Bronsted-Lowry theory defines acids as proton donors and bases as proton acceptors. Eq
0 views • 29 slides
Understanding Hard and Soft Acids and Bases in Chemistry
Explore the concepts of Hard and Soft Acids and Bases (HSAB) in chemistry, examining the relative strengths of acids in solution, stabilities of complexes, predictions of reaction courses, and the separation of cations based on HSAB rules. Discover how the interactions between different acid-base co
1 views • 14 slides
Understanding Hard and Soft Acids and Bases (HSAB Principles) by Dr. Gurpreet Kaur
Delve into the world of Hard and Soft Acids and Bases (HSAB) with Dr. Gurpreet Kaur as she explains the characteristics of hard and soft acids, Pearson's HSAB principle, applications such as predictions of coordination in complexes, poisonings of metal catalysts, and the classification of acids and
2 views • 17 slides
Understanding Acids and Bases: pH Scale Explained
Exploring the pH scale, this content delves into the fundamentals of acidity and alkalinity, covering what pH stands for, the inventor of the pH scale, reactions when acids and bases combine, and where the weakest acids and bases are found on the pH scale.
0 views • 7 slides
Understanding Amino Acids: Qualitative Tests and Properties
Amino acids play a crucial role as building blocks of proteins and can be converted into specialized products. There are 20 common L-α-amino acids found in mammalian proteins, each with a unique structure and classification based on their side chain properties. Amino acids exhibit optical activity,
1 views • 34 slides
Understanding Acids, Bases, and Neutrals in Natural Sciences Grade 7
Acids, bases, and neutrals are vital substances found in various settings like factories and laboratories. They exhibit distinct properties, with acids feeling rough, being corrosive, and containing hydrogen ions, while bases feel slippery, taste bitter, and contain hydroxide ions. While acids like
0 views • 15 slides
Understanding Acids and Bases: Definitions and Behavior
Explore the definitions of acids and bases according to Arrhenius and Brønsted-Lowry theories, their behavior at a particulate level, and the differences between strong and weak acids and bases. Learn how to define, identify, and understand the properties of acids and bases through practical exampl
0 views • 12 slides
The Intriguing Structure and Functions of DNA
DNA, or Deoxyribonucleic Acid, is a fundamental molecule in living organisms, characterized by its double helical structure consisting of two antiparallel polynucleotide chains. Each strand is composed of nucleotide monomers, comprising deoxyribose sugar, phosphate group, and nitrogenous bases (puri
0 views • 12 slides
Amino Acid: Structure and Classification Overview
Amino acids are essential building blocks of proteins, with only 20 out of the 300 occurring in nature being used for protein synthesis. The structure of amino acids consists of four groups attached to a central carbon atom. At physiological pH, these groups can ionize, forming zwitterions. Amino ac
0 views • 24 slides
Understanding Buffer Solutions: Properties and Applications
Buffer solutions are essential in resisting pH changes and consist of mixtures of weak acids or bases with their salts. They maintain constant pH levels despite dilution or addition of acids/bases. Buffer capacity measures the amount of acid/base needed for a pH change. Biological systems and pharma
3 views • 4 slides
Understanding Ointments and Ointment Bases in Pharmaceutics
In the field of pharmaceutics, ointments play a crucial role as semisolid dosage forms for topical application. They serve as vehicles for medicinal substances, offering protective and emollient functions. Ointments are composed of ointment bases, which can be medicated or non-medicated, providing d
0 views • 31 slides
Understanding Buffer Solutions and pH Indicators
Buffer solutions resist changes in pH upon the addition of acids or bases, maintaining a stable pH level. They consist of a weak acid and its conjugate base or a weak base and its conjugate acid. pH indicators are weak acids or bases that exhibit color changes based on their degree of dissociation a
0 views • 21 slides
Understanding Amino Acids: Structure, Classification, and Properties
Amino acids are crucial chemical units that form proteins and play essential roles in the body. They consist of a carboxyl group and an amino group, serving as building blocks and metabolic intermediates. This article covers the general structure, zwitterions, isoelectric point, pK values, and class
0 views • 26 slides
Understanding Buffers in Chemistry
The concept of buffers in chemistry plays a crucial role in maintaining stable pH levels in solutions. Buffers consist of components that neutralize acids and bases, helping prevent drastic pH changes. Weak acids or bases are ideal buffer components due to their ability to react with both acids and
1 views • 15 slides
Introduction to Analytical Chemistry and pH Detection
Analytical chemistry involves the study of detecting acids and bases in solutions, with methods such as litmus testing and the Arrhenius definition. The concept of conjugate acids and bases, acid dissociation, and buffer solutions are also key components in understanding chemical properties and reac
1 views • 36 slides
Understanding Acids and Bases in Chemistry
Acids donate protons, while bases accept them. Strong acids ionize completely while weak acids only partially ionize, resulting in a Ka value less than one. Water, being amphoteric, can both donate and accept protons. The ionization of water leads to a constant Kw value of 10^-14. Explore the ioniza
0 views • 15 slides
Qualitative Tests of Proteins & Amino Acids: Overview and Analysis
In this lab, you will delve into the qualitative tests for proteins and amino acids, understanding their structures, classifications, and importance in food and human nutrition. The tests include solubility tests and identification tests for both amino acids and proteins, revealing their presence an
0 views • 13 slides
Qualitative Tests of Proteins & Amino Acids - Lab Analysis Overview
This lab analysis covers qualitative tests for proteins and amino acids, including solubility tests and identification tests for amino acids and proteins. Specific tests like Ninhydrin test for -L amino acids, Xanthoproteic test for aromatic amino acids, and lead sulfite test for sulfhydryl group de
0 views • 13 slides
Comparison of Aliphatic and Aromatic Carboxylic Acids in Chemical Properties
The article explores the differences between aliphatic and aromatic carboxylic acids, detailing their properties, common uses in food and cosmetics, as well as their reactions in ignition tests. It discusses the appearance changes and odor variations upon ignition, along with the distinctive color f
0 views • 14 slides
Understanding Acids and Bases: A Comprehensive Overview
Learn about the fundamental concepts of acids and bases, their classifications, and key theories by Arrhenius and Brønsted-Lowry. Explore the importance of pH balance in living organisms and how different substances exhibit acidic or basic properties through informative visuals.
0 views • 34 slides
Understanding Analytical Chemistry Concepts and Applications
In the realm of analytical chemistry, various methods are employed to detect acids and bases in solutions. Litmus, a natural dye, is commonly utilized for this purpose, turning red in acidic conditions and blue in basic conditions. The slides presented touch upon key concepts such as the Arrhenius d
0 views • 47 slides
Understanding Hard and Soft Acids and Bases Theory
Dive into the world of Hard and Soft Acids and Bases (HSAB) theory which explains the interaction preferences between acids and bases based on their properties. This theory enhances our understanding of chemical reactions, helping predict favorable outcomes and shed light on reactions that classic t
0 views • 12 slides
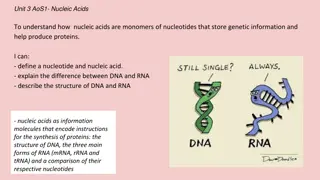
Understanding Nucleic Acids: DNA, RNA, and Nucleotides
Nucleic acids are essential molecules that store genetic information and aid in protein production. This content delves into the monomeric structure of nucleotides, the differences between DNA and RNA, the unique characteristics of DNA's double helix structure versus RNA's single-stranded form, and
0 views • 11 slides
Understanding Nucleic Acids: DNA, RNA, and Nucleotides
Nucleic acids, essential for all organisms, store genetic information as long polymer chains of nucleotides. Nucleosides contain bases and sugars, while nucleotides include a phosphate. Purines and pyrimidines are the aromatic bases in nucleotides, with DNA having A, G, C, T and RNA having A, G, C,
0 views • 10 slides
Understanding Acids and Bases in Chemistry
Acids and bases play essential roles in chemistry, where they release hydrogen ions or hydroxide ions when mixed with water. Acids, like vinegar and lemon juice, are corrosive and can cause chemical burns. On the other hand, bases, such as bleach and dish soap, contain the hydroxide group in their f
0 views • 19 slides
Understanding Conjugate Acids and Bases in Chemistry
Explore the concept of conjugate acids and bases in chemistry through definitions, reactions, and examples. Learn how to identify strong and weak acids/bases based on their conjugates and understand the behavior of acids/bases in reverse reactions. Discover the significance of conjugate acids/bases
0 views • 22 slides
Understanding Acids and Bases: The Fundamentals Explored
Explore the foundational concepts of acids and bases, from Arrhenius to Lewis definitions. Delve into how these theories explain chemical reactions and properties, highlighting the significance of proton transfer and electron interactions in determining acid-base behavior.
0 views • 29 slides
Understanding pH Calculations for Acids and Bases in Water
Acids and bases play crucial roles in chemical reactions by involving the transfer of protons. This lecture explains the definitions of acids and bases, general acid-base reactions in water, and how the pH scale is used to measure the acidity or basicity of a solution. It covers how acids and bases
0 views • 42 slides
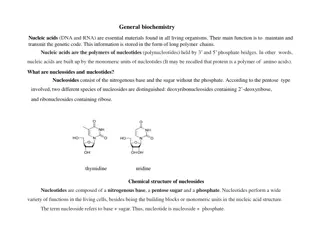
Understanding Nucleic Acids: The Building Blocks of Life
Nucleic acids, such as DNA and RNA, play a crucial role in storing and transmitting genetic information in living organisms. These biopolymers are composed of nucleotides, each consisting of a sugar, phosphate group, and nitrogenous base. DNA and RNA differ in sugar composition and nucleobases. Nucl
0 views • 14 slides
Understanding Acids and Bases: Ionization and Properties
Acids donate protons while bases accept them. Strong acids and bases ionize completely, while weak acids ionize partially. Water is amphoteric, capable of both accepting and donating protons. The equilibrium constant Kw for water is 10^-14. Understanding the ionization of weak acids and weak bases h
0 views • 15 slides
Understanding Acid-Base Chemistry Theories and Buffers
Explore the concepts of acids, bases, and buffers through various theories such as Arrhenius, Lowery-Bronsted, and Lewis. Learn about the limitations of the Arrhenius theory and delve into different types of buffer solutions that help resist pH changes. Gain insights into the definitions, examples,
4 views • 36 slides
Understanding Polyprotic Acids and Salts in Chemistry
Exploring the concept of polyprotic acids, particularly focusing on sulfuric acid as a unique example with its dual dissociation steps. The discussion delves into the equilibrium concentrations of ions in solution, calculating pH values for different acid concentrations, and understanding the signif
0 views • 18 slides
Understanding Acids and Bases in Chemistry: Key Concepts and Terminology
This chapter delves into the fundamental concepts of acids and bases in chemistry, covering Arrhenius, Bronsted-Lowry, and Lewis definitions. It discusses conjugate acids and bases, acid dissociation, dissociation of strong and weak acids, and the differences between strong and weak acids/bases. The
0 views • 41 slides
Exploring the World of Nucleotides and Nucleic Acids
Discover the fascinating realm of nucleotides and nucleic acids through characteristic bases, pentoses, tautomeric forms of common pyrimidine and purine bases, electron-rich nature, UV absorption spectra, furanose structures, major and minor bases, nucleosides, and phosphodiester bonds linking succe
0 views • 26 slides
Understanding Nucleic Acids and the Central Dogma of Molecular Biology
In molecular biology, understanding nucleic acids like DNA and RNA is crucial for comprehending the central dogma, which explains the flow of genetic information from DNA to RNA to proteins. Nucleic acids are polymers of nucleotides, composed of pentose sugar, nitrogenous bases, and phosphate groups
0 views • 18 slides
Understanding Acids, Bases, and Indicators in Grade 7 Natural Sciences
This presentation covers the topic of acids, bases, and neutrals in Grade 7 Natural Sciences. It explains the use of acid-base indicators, such as litmus paper, and how they react to different substances. The content also discusses how certain detergents and soaps act as bases and how the soil pH af
0 views • 6 slides
Understanding Hard & Soft Acids and Bases in Chemistry
Explore the concepts of hard and soft acids and bases in chemistry, including the Arrhenius, Brønsted-Lowry, Lewis, and Solvent System models. Learn about the HSAB principle introduced by R.G. Pearson, discussing polarizing power and polarizability of acids and bases. Understand how these principle
0 views • 15 slides
Understanding Acids and Bases in Chemistry
Acids, derived from the Latin word "acidus" meaning sour, produce hydrogen ions when dissolved in water. Bases, defined as compounds dissociating into metal ions and hydroxide ions in water, have common characteristics like a bitter taste. The Brønsted-Lowry theory expanded the definitions of acids
1 views • 50 slides