Canizzaro Reaction in Organic Chemistry: Experiment and Applications
The Canizzaro reaction involves the disproportionation of aldehydes in the presence of a strong base to produce an alcohol and a carboxylic acid. This experiment, supervised by Lecturer Israa Radhi, explores the mechanism and practical application of the reaction. Benzyl alcohol and benzoic acid, pr
1 views • 7 slides
Cannizzaro Reaction: Separation of Benzoic Acid and Benzyl Alcohol
The Cannizzaro reaction involves the separation of benzoic acid and benzyl alcohol by utilizing various reagents and procedures such as dissolving potassium benzoate, washing with ether, and treatment with different solutions to isolate the desired compounds. The process involves multiple steps incl
7 views • 4 slides
Understanding the Diels-Alder Reaction in Practical Organic Chemistry
The Diels-Alder reaction is a fundamental method in organic chemistry for producing cyclic organic compounds by combining a conjugated diene with an alkene. This reaction, named after Otto Diels and Kurt Alder, involves the formation of a six-membered ring with specific bond rearrangements. Conjugat
4 views • 15 slides
Cannizzaro Reaction
The Cannizzaro reaction is a chemical reaction involving the base-induced disproportionation of non-enolizable aldehydes to form a primary alcohol and a carboxylic acid. Discover more about this reaction, its history, mechanism, and variants like the Cross Cannizzaro reaction and Intramolecular Cann
1 views • 20 slides
Organometallic Chemistry III: Transition Metal Complexes and Homogeneous Catalysis
Explore the reactivity of transition metal complexes, including bond metatheses and various reactions. Learn about orbital considerations, synthesis, and spectroscopic properties of organometallic complexes. The course covers basics from AC1, focusing on ligands, electron counting, and MO diagrams.
5 views • 8 slides
Benzoin Condensation: A Name Reaction Explained by Dr. Atul Kumar Singh
Benzoin condensation is a classic organic reaction where aromatic aldehydes self-condense to form α-hydroxy ketones. Dr. Atul Kumar Singh, an Assistant Professor of Chemistry, details the mechanism and the specific catalytic properties of cyanide in this reaction. The reaction involves refluxing th
0 views • 6 slides
Investigating Impact of Practice on Human Reaction Time Through Ruler Drop Test
This practical investigation focuses on determining if practice can reduce human reaction times by conducting a ruler drop test. Participants use their weaker hand to catch a ruler dropped by their partner, aiming to improve their reaction time with practice. The experiment explores how athletes can
0 views • 7 slides
Understanding Favorskii Rearrangement in Organic Chemistry
Favorskii rearrangement is a base-catalyzed rearrangement reaction of halo ketones giving rise to acid, ester, or amine compounds via a cyclopropane intermediate. The mechanism, evidence supporting it, variations in reaction based on the presence of hydrogen, and stereospecificity are discussed with
0 views • 8 slides
Industrial Production of Citric Acid: Fermentation Processes and Applications
Citric acid is industrially produced through microbial fermentation processes. It is widely used in various industries such as food, pharmaceuticals, and beverage production. Different methods like surface culture fermentation are employed for citric acid manufacture, with fungi like Aspergillus nig
4 views • 18 slides
Limit Test of Iron Based on Color Reaction with Thioglycollic Acid
The limit test for iron involves the reaction of iron in ammoniacal solution with citric acid and thioglycollic acid to form a reddish-purple color. By comparing the color produced with a standard solution, the presence of iron is determined. Citric acid prevents precipitation of iron, while thiogly
1 views • 5 slides
Understanding Biosynthetic Pathways in Living Organisms
Biosynthesis, also known as anabolism, involves the formation of complex organic compounds from simple subunits catalyzed by enzymes within living organisms. This process is vital for the development of life and the production of essential compounds like carbohydrates, proteins, vitamins, antibiotic
1 views • 21 slides
Kinetic Reaction of Sulphite and Iodate - Landolt Reaction Overview
The kinetic reaction of sulphite ions and iodate in the Landolt reaction is a fascinating chemical process where slow and fast reactions occur sequentially, resulting in a visually striking color change. By monitoring the induction period between the two reactions, one can observe the formation of h
0 views • 9 slides
Determination of Acetic Acid Content in Vinegar Experiment
The experiment aims to measure the total acid concentration in a specific brand of vinegar through a titration process using NaOH solution. The procedure involves titrating the vinegar solution with NaOH until a pink color appears, calculating the concentration of acetic acid, and determining the pe
2 views • 8 slides
Exploring Enzyme Kinetics for Understanding Chemical Reactions
Enzyme kinetics is a vital discipline focusing on the rate of enzyme-catalyzed reactions and how they respond to varying conditions. Reactions are classified based on reactant concentration influences. Zero, first, second, and third order reactions are distinguished, with examples like first-order r
0 views • 31 slides
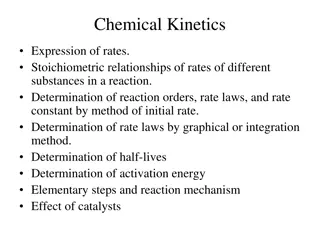
Understanding Chemical Kinetics: Rates, Reactions, and Mechanisms
Chemical kinetics involves studying reaction rates, rate laws, stoichiometry, and factors affecting reaction speed. This branch of chemistry delves into determining reaction orders, rate constants, and activation energies using various methods. Different types of rates, such as initial, instantaneou
2 views • 68 slides
Understanding the Prins-Pinacol Reaction in Organic Chemistry
The Prins-Pinacol reaction involves a two-step process starting with the Prins reaction and followed by the Pinacol rearrangement. This reaction, discovered in 1919 by Hendrick J. Prins, is a crucial transformation in organic chemistry, leading to the formation of important carbonyl compounds. The m
0 views • 14 slides
Understanding Kinetics and Reaction Rates in Chemistry
Kinetics is the study of reaction rates and factors affecting them, such as concentration, temperature, catalysts, and more. Orders of reaction classify reactions based on rate dependency on reactant concentration. Factors like pH, light, and solvents can also impact reaction rates. Half-life and sh
0 views • 18 slides
Synthesis of Salicylic Acid: Theory, Derivatives, and Applications
Salicylic acid is synthesized from methyl salicylate through ester hydrolysis with aqueous alkali. It is a versatile compound used in organic synthesis, as a plant hormone, and derived from salicin metabolism. The derivatives of salicylic acid can minimize gastric disturbances and enhance therapeuti
4 views • 12 slides
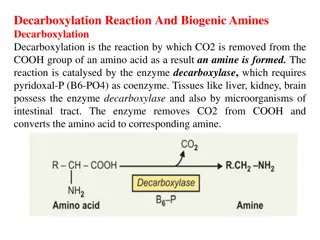
Decarboxylation Reaction and Biogenic Amines in Amino Acid Metabolism
Decarboxylation is a crucial reaction in amino acid metabolism where CO2 is removed to form biogenic amines, catalyzed by decarboxylase enzymes. Important biogenic amines include tyramine, tryptamine, and histamine, each impacting physiological functions like blood pressure regulation. Aromatic amin
0 views • 25 slides
Overview of Salicylic Acid and its Derivatives
Salicylic acid is known for its antiseptic, germicidal, and analgesic properties, used in various applications such as preservatives, wart treatments, and pain relief. Different derivatives of salicylic acid are developed to minimize side effects and enhance efficacy. Acetyl salicylic acid (ASA), co
0 views • 15 slides
Understanding Chemical Kinetics: Reaction Rates and Mechanisms
Chemical kinetics is a branch of chemistry focused on studying reaction rates and mechanisms. Unlike thermodynamics, which deals with feasibility, kinetics explores the speed at which reactions occur. Factors such as temperature, pressure, and catalysts influence reaction rates. Understanding the ra
3 views • 72 slides
Advances in sp3C-H Activation Catalyzed by Palladium - Bo Yao
Recent progress in the field of sp3C-H activation catalyzed by palladium has led to significant insights and challenges. Concepts related to C-H activation, strategies employed, and identified obstacles are discussed, highlighting the importance of this area in catalysis research.
0 views • 15 slides
Utilizing a Global Model for Analyzing Reaction Pathways in Plasma Systems
This research focuses on using a kinetic global model framework to identify relevant reactions in chemically complex plasma systems. The framework, KGMf, enables the investigation of macroscopic plasma characteristics by analyzing reaction pathways, sensitivity to reaction rate errors, and dominant
1 views • 6 slides
Understanding Free Energy, Reaction Quotient, and Equilibrium Constant
This educational material delves into the concepts of free energy, reaction quotients, and equilibrium constants in chemical systems. It explains how to determine the direction of a reaction based on Q and K values, elucidates the role of Gibbs free energy in determining spontaneity, and provides ca
0 views • 10 slides
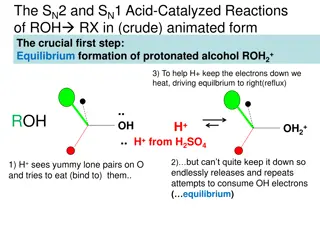
Acid-Catalyzed Reactions of Alcohols: SN2 and SN1 Mechanisms
The acid-catalyzed reactions of alcohols involve SN2 and SN1 mechanisms where protonated alcohols react with RX. In the SN2 mechanism, a nucleophile attacks a protonated 1o alcohol, leading to inversion. On the other hand, in the SN1 mechanism, a carbocation intermediate is formed, and a nucleophile
0 views • 12 slides
Palladium-Catalyzed Arylhalogenation of Alkenes: Seminar Highlights
Seminar conducted by Jian Cao from Jieping Zhu's Lab at EPFL on the topic of Palladium-Catalyzed Arylhalogenation of Alkenes, covering Pd(II)/Pd(IV) and Pd(0)/Pd(II) catalytic cycles, stereochemical studies, historical development, and comprehensive research findings.
0 views • 42 slides
Transition Metal Catalyzed Trifluoromethylation and Its Importance in Pharmaceutical and Material Science
Transition metal catalyzed trifluoromethylation is a valuable method for introducing trifluoromethyl groups into unactivated alkenes, with significant impact in pharmaceutical, agrochemistry, and material science. The incorporation of CF3 is essential due to its enhancement of metabolic stability, p
0 views • 24 slides
Understanding Purine Degradation and Gout
Purine degradation pathway involves the breakdown of dietary nucleic acids, mainly from meat, into uric acid through specific enzymatic steps. Excessive uric acid production can lead to conditions like gout and hyperuricemia. Humans excrete uric acid in the urine as the final product, while other an
1 views • 12 slides
Grignard Reaction in Chemistry Lab: Part 1 Overview
The Grignard Reaction Part 1 in Chemistry 318 Fall 2018 involves the preparation of the Grignard reagent, its reaction with CO2, and the isolation of the benzoic acid product. The experiment spans two lab sessions, focusing on safety precautions, pre-lab checks, and upcoming due dates. Students are
0 views • 11 slides
Introduction to Chemical Reaction Engineering (CRE)
Chemical Reaction Engineering (CRE) focuses on studying the rates and mechanisms of chemical reactions, as well as designing reactors for these reactions. The field involves understanding balances in terms of molar flow rates, mole balances, rate laws, stoichiometry, and membrane reactors. Membrane
0 views • 20 slides
Understanding Factors Affecting Enzyme Activity in Biochemistry
Enzyme assays measure substrate conversion to product under varying conditions like cofactors, pH, and temperature. Enzyme velocity represents the rate of a catalyzed reaction, typically reported as V0. Enzyme activity is expressed as mol of substrate transformed per minute, with enzyme unit and kat
0 views • 18 slides
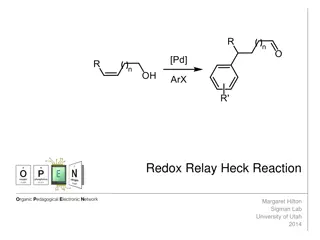
Understanding the Redox-Relay Heck Reaction in Organic Synthesis
The Redox-Relay Heck Reaction is a powerful tool in organic synthesis that allows for the functionalization of olefins with aryl groups. Developed by Sigman and colleagues, this reaction involves a palladium-catalyzed relay controlled by a thermodynamic sink, leading to the formation of aldehydes or
0 views • 6 slides
Understanding Chemical Kinetics: Reaction Rates and Activation Energy
Exploring the fundamental concepts of chemical kinetics, this content delves into reaction rates, collision theory, and activation energy in chemical reactions. It emphasizes the importance of particle collisions, correct orientation, and energy requirements for reactions to occur. Through energy di
0 views • 17 slides
Organic Chemistry: Aldol Condensation Experiment Overview
Organic chemistry students learn about the Aldol condensation reaction involving ketones and aldehydes. The experiment involves the reaction of acetone with benzaldehyde catalyzed by sodium hydroxide to form a trans, trans-isomer. The reaction is illustrated step by step, from the formation of the e
0 views • 11 slides
Common Organic Acids and Their Uses in Various Industries
Citric acid, lactic acid, salicylic acid, and tartaric acid are natural acids found in various fruits. They have diverse applications in food and beverage, pharmaceutical, and cosmetic industries. These acids are used for flavoring, cleaning, as food additives, and in the preparation of various prod
0 views • 4 slides
Insights into Transition Metal Catalyzed Olefin Polymerization Mechanisms
Detailed exploration of transition metal catalyzed olefin polymerization via coordination-insertion mechanism, including the use of Ziegler-Natta catalysts, MAO activators, and stereochemical control of poly-1-alkenes. The mechanism, catalyst types, activators, and stereochemistry effects are discus
0 views • 19 slides
Synthesis of Banana Oil in Chemistry Class
The synthesis of banana oil involves a reaction between carboxylic acid and alcohol catalyzed by H2SO4 to produce isoamyl acetate. The mechanism includes nucleophilic acyl substitution with two main reaction steps - addition and elimination. The process shifts the equilibrium to favor product format
0 views • 16 slides
Understanding Serum Uric Acid Levels and Hyperuricemia
Serum uric acid is a crucial marker of purine metabolism, with elevated levels indicating hyperuricemia which can lead to conditions like Gout. The measurement of serum uric acid helps in diagnosing hyperuricemia, with serum being the preferred specimen for testing. Various factors such as diet, gen
0 views • 12 slides
Understanding Kinetics in Chemical Reactions
Kinetics is the study of reaction rates and factors affecting them. Reaction rate is the speed at which a reaction occurs, influenced by factors like concentration, temperature, pH, light, catalysts, and solvents. Reactant concentration determines reaction order, which categorizes reactions as zero-
0 views • 18 slides
Understanding Fatty Acid Metabolism in Animals
Animals cannot convert fatty acids into glucose due to the inability to synthesize glucose from fatty acids. The process involves acetyl-CoA not being converted into pyruvate or oxaloacetate, leading to the citric acid cycle and differences between fatty acid synthesis and degradation pathways. Key
0 views • 8 slides