The Intriguing Element of Francium: Discovery and Properties
Francium is a radioactive metal with the atomic number 87, discovered by Marguerite Perey in 1939. It is the second rarest naturally occurring element and exhibits unique properties due to its instability. Despite being challenging to study, Francium's discovery sheds light on its intriguing characteristics and historical significance in the periodic table.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
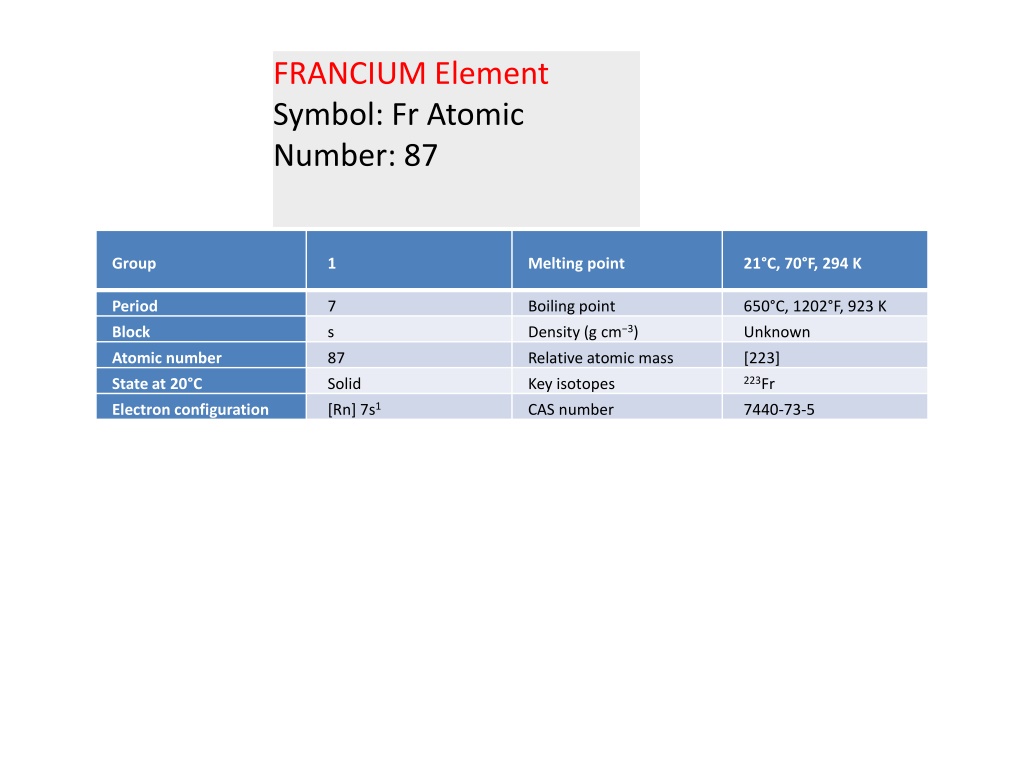
FRANCIUM Element Symbol: Fr Atomic Number: 87 Group 1 Melting point 21 C, 70 F, 294 K Period 7 Boiling point Density (g cm 3) 650 C, 1202 F, 923 K Block s Unknown Atomic number 87 Relative atomic mass [223] 223Fr State at 20 C Solid [Rn] 7s1 Key isotopes Electron configuration CAS number 7440-73-5
Francium (previously known as eka-cesium and actinium K) is a radioactive metal and the second rarest naturally occurring element after Astatine. It is the least stable of the first 103 elements. Very little is known of the physical and chemical properties of Francium compared to other elements. Francium was discovered by Marguerite Perey of the Curie Institute in Paris, France in 1939. However, the existence of an element of atomic number 87 was predicted in the 1870s by Dmitri Mendeleev, creator of the first version of the periodic table, who presumed it would have chemical and physical properties similar to Cesium. Several research teams attempted to isolate this missing element, and there were at least four false claims of discovery during which it was named Russium (after the home country of soviet chemist D. K. Dobroserdov), Alkalinium (by English chemists Gerald J. K. Druce and Frederick H. Loring as the heaviest alkali metal), Virginium (after Virginia, home state of chemist Fred Allison), and Moldavium (by Horia Hulubei and Yvette Cauchois after Moldavia, the Romanian province where they conducted their work). Perey finally discovered Francium after purifying radioactive Actinium-227 from Lanthanum, and detecting particles decaying at low energy levels not previously identified. The new product exhibited chemical properties of an alkali metal (such as co-precipitating with Cesium salts), which led Perey to believe that it was element 87, caused by the alpha radioactive decay of Actinium-227. The International Union of Pure and Applied Chemistry officially adopted the name Francium in 1949, becoming the second element (after Gallium) to be named after France
Pereys discovery has been described as accidental, and simply attributed to an anonymous assistant of Marie Curie. However, whilst Perey began working as Marie Curie s personal assistant, she was an independent radiochemist at the time of discovery, which occurred five years after Curie s death. Perey s scientific publications on the discovery of Francium outline the painstaking work she undertook, carrying out analysis of hundreds of fractional crystallisations. Marguerite Perey was the first woman to be elected to the Acad mie de Sciences (French Academy of Sciences), was Professor and Chair of the Department of Nuclear Chemistry at the University of Strasbourg, but succumbed in 1975 to cancer contracted through her research at age 65. There are 34 known isotopes of Francium ranging in atomic mass from 199 to 232. Francium-223 and Francium- 221 are the only isotopes that occur in nature, though the former is the most common. Francium is the most unstable of the naturally occurring elements: its most stable isotope, Francium-223, has a half-life of only 22 minutes.
Francium can also be synthesised in a nuclear reaction by combining isotopes of Gold and Oxygen, with the largest amount produced in the laboratory being a cluster of over 300,000 atoms. In this case, enough Francium was trapped to enable a video camera to capture the light given off by the atoms as they fluoresce, appearing as a glowing sphere about 1 millimetre in diameter. This was the very first time that anyone had ever seen Francium. Francium has yet to be synthesised in amounts large enough to weigh. Due to its instability and rarity, there are no commercial applications for Francium. However, it is used for research purposes to investigate atomic structure. Francium s relatively simple atomic structure and large atomic mass and number have made it the subject of specialised spectroscopy experiments that have led to more specific information regarding energy levels and the coupling constants between subatomic particles
Francium was the most brain stretching of my three elements. I was stimulated and highly motivated to try and absorb the idea that Francium is so rare that as little as 20-30 grams exists at any given time throughout the earth s crust. It is the second rarest naturally occurring element after astatine. With this in mind I tried to produce an image that had complex intertwining colours in an attempt to express the idea of how unique this element is.
Isotopesfrancium francium-223 Francium-224 Francium-221 Francium-222 francium-226 francium-225 Francium-220 Francium-210 Francium-228 Francium-212 Francium-227 francium-232 Francium-216 Francium-214 Francium-217 Francium-215
Reaction of francium with air So far as I know, nobody has ever assembled enough francium in one place to know for certain its appearance. It is probably a very soft, easily cut, solid metal, perhaps even a liquid at room temperature. A fresh francium surface would soon tarnish because of reaction with oxygen and moisture from the air. Were francium ever to be burned in air, the result is expected to be francium superoxide, FrO2. Fr(s) + O2(g) FrO2(s)
Reaction of francium with water Francium is very scarce and expensive. It is umlikely that anyone has ever reacted the metal with water. However, given that all the other Group 1 elements react to form colourless solutions of the hydroxide and hydrogen gas (H2), it would be strange were francium not to do the same. The resulting solution would be basic because of the dissolved hydroxide. The reaction would probably sbe faster than that of caesium - in other words dangerously quick. 2Fr(s) + 2H2O(l) 2FrOH(aq) + H2 Reaction of francium with the halogens So far as I know, nobody has ever assembled enough francium in one place to carry out its reactions with halogens. However one can predict that francium metal would react vigorously with all the halogens to form francium halides. So, it would reacts with fluorine, F2, chlorine, Cl2, bromine, I2, and iodine, I2, to form respectively francium(I) bromide, FrF, francium(I) chloride, FrCl, francium(I) bromide, FrBr, and francium(I) iodide, FrI. 2Fr(s) + F2(g) FrF(s) 2Fr(s) + Cl2(g) FrCl(s) 2Fr(s) + Br2(g) FrBr(s) 2Fr(s) + I2(g) FrI(s)
Reaction of francium with acids I'm not sure this reaction has ever been done, but one would predict that francium metal will dissolve very readily in dilute sulphuric acid to form solutions containing the aquated Fr(I) ion together with hydrogen gas, H2. 2Fr(s) + H2SO4(aq) 2Fr+(aq) + SO42-(aq) + H2(g)
Reaction of francium with bases Francium is very scarce and expensive. It is umlikely that anyone has ever reacted the metal with water. However, given that all the other Group 1 elements react to form colourless basic solutions of the hydroxide and hydrogen gas (H2), it would be strange were francium not to do the same. The reaction would continue even when the solution becomes basic. The resulting solution would be basic because of the dissolved hydroxide. The reaction would probably sbe faster than that of caesium - in other words dangerously quick. 2Fr(s) + 2H2O(l) 2FrOH(aq) + H2