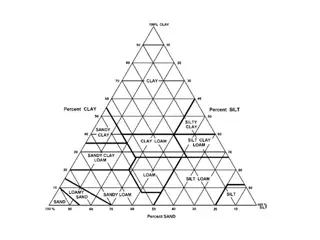
Comprehensive Soil Cation Exchange Capacity (CEC) Measurement Lab Procedure
This lab exercise focuses on measuring CEC using soil samples, extracting exchangeable cations, analyzing basic cations through AA spectroscopy, titrating acid cations, calculating CEC and base saturation percentage for the soil, and converting cation measurements from meq/100g to lbs/acre. The step-by-step procedure involves steps like weighing soil samples, shaking with NaCl solution, analyzing exchangeable cations, titrating acids, and performing calculations. Image references and systematic measurements guide the process from start to finish.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Lab 6: Measuring CEC Use soils from last week to > extract exchangeable cations > measure basic cations by AA spectroscopy > measure acid cations by titration > calculation CEC, % BS for your soil > convert from meq/100g to lbs/acre
Procedure: 1. Weigh 3.0 g soil into 50 mL centrifuge tube 2. Pump in 35 mL NaCl extracting solution 3. Shake for 20 minutes to displace exchangeable cations from colloid surface into solution
Ion Exchange by Added Na soln Mass Action Mg+2 Ca+2 K+ Al+3 Displaced cations
Measurement of exchangeable cations: 1. Centrifuge, filter to separate clear solution. 2. Fill glass vial about full of solution for AA analysis. 3. Measure remaining solution using grad cylinder record this number, this will be your volume titrated . Pour back into Erlenmeyer flask.
Titration of exchangeable acids: 1. To Erlenmeyer flask: add stir bar and 3-4 drops phenolpthalein indicator , turn on stirrer to slow speed. 2. Add NaOH from burette SLOWLY (drop by drop) until solution stays pink for 20-30 seconds. Record volume base used from burette. At endpoint: pH is near neutral, and AMT BASE ADDED = AMT ACID PRESENT NB x VB = NA x VA
Titration calculations: NB: conc of base: 0.005 N (eq/L = meq/mL) VB: volume of base (mL, burette) VA: volume of acid ( mLs titrated ) NA: conc of acid: UNKNOWN meq acid/mL NB x VB = NA x VA
Conc (solution) Conc (soil) We used 35 mL of NaCl solution to extract 3 g soil to get cations into solution . Meq acid x 35 mL soln x 100 = meq acid mL soln 3 g soil 100 100 g soil Same for basic cations by AA: Meq Ca x 0.035 L soln x 100 = meq Ca L soln 3 g soil 100 100 g soil
CEC of soil = cations (in meq/100 g): Ca + Mg + K + acidity (H+Al) Mg and K: written on board (meq/L) .. % base saturation (BS) = bases (Ca+Mg+K)/CEC Convert meq/100 g into lbs/acre-furrow slice .
CEC calculations: meq/100 g to lbs/acre Assume a soil has 2 meq K+/100 g: how many lbs/acre? ( acre = acre-furrow slice= 2x106 lbs) Note: Atomic mass of K=39 g/mole, and 1mol=1equiv (K+1) X 1 mol 39 K g mol 1 equivX 1000 mg 1 equiv 1000 meq = 39 mg meq X 1 g So-- 780 mg K kg soil 1000 g 1 kg 39 mg K 1 meq 2 100 soil g meq K = x x Now: mg/kg is PPM = parts per million and LBs/AFS = lbs/2x106lbs, which is parts per 2 million . SO: 780 ppm K x 2 = 1560 lbs K/acre