Exploring the Fascinating World of Elements
Dive into the intriguing characteristics of different elements, from the peculiarities of Hydrogen to the inert nature of Helium, the reactivity of Lithium and Beryllium, the uniqueness of Boron, and the importance of Carbon in organic chemistry. Discover their properties, electron configurations, and bonding behaviors, shedding light on the fundamental building blocks of the universe.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
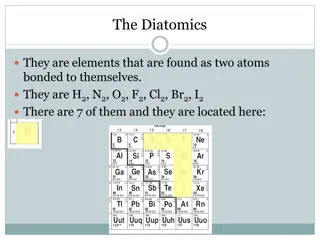
The Elements Start with some, learn more.
1.00794 +1 H 1 1 Hydrogen -1 The weirdest element. It s in group 1, but it s not a metal. It bonds like a nonmetal (covalent) but in water, hydrogen compounds like HCl or H2SO4 form ions, which are ACIDS. It s in period 1, it has one orbital, with 1 electron. Almost all hydrogen is H-1, but there are two isotopes in our class, H-2 with one neutron, and H-3, with two neutrons. It exists naturally as H2, a diatomic molecule, not as single atoms. Single atoms of hydrogen are unstable. It s a HONClBrIF twin.
4.00260 0 He 2 2 Helium The dullest element. Totally inert, unreactive. It s in group 1, and it s a noble gas. It does not bond with anything, ever. It s very much less dense than air, so balloons of it float and make kids smile. It s in period 1, it has one orbital, with 2 electron. That is a FULL orbital, classic for noble gases.
6.941 +1 Li 3 2-1 Lithium A fairly reactive metal, it s the first element in Period 2, which means it has TWO orbitals. The first one is full and the last (valence) electron starts the second orbital. It makes ONLY +1 cations. That means it LOSES one electron when making ionic bonds. Lithium is used in batteries because that valence electron is readily shed (forming the electricity) in a battery. Group 1 metals are called Alkali metals because when put into water they form alkaline solutions (another vocabulary word that means bases).
9.01218 +2 Be 4 2-2 Beryllium This is the second metal in period 2, which means it has two orbitals. When it becomes a cation in an ionic bond it must lose TWO electrons, becoming a Be+2 cation. It loses 2 electrons and becomes ISOELECTRIC to helium. Beryllium is the first metal in group 2, which are called the Alkaline Earth Metals. (vocabulary)
10.81 +3 B 5 2-3 Boron This is our second WEIRDO element. Boron is a nonmetal AND a metalloid as well. In our class it will NOT make ions. It does have an oxidation state of +3 so it will make covalent bonds with other nonmetals. Boron is on the regents nearly every time, because of its electron configuration. 2 electrons plus 3 electrons. Not complicated, but many kids get tricked when you need to draw the Lewis Dot Diagram . In those you ONLY show valence electrons, but the grand total of five electrons could fit into the outermost orbital (IF THEY WERE THERE!). Don t get tricked. Boron rhymes with moron too. With 3 valence electrons, only draw 3 dots in the Lewis diagram!
12.011 +4 C 6 2-4 Carbon +2 +4 Probably the most important atom for living things, carbon is the CENTRAL atom in organic chemistry. All organic compounds start with carbon. Carbon makes no ions in our class. Carbon MUST make 4 covalent bonds. It can make single, double and triple bonds to another carbon atom, or other atoms too. It can make chains and rings of atoms. Carbon is a solid, it s NOT carbon dioxide, which is a compound. One twelveth of a C-12 isotope is the mass of one amu, it s the reason that Carbon is in the KEY of your periodic table.
12.011 -3 -2 -1 +1 +2 +3 +4 +5 Nitrogen This is the most common gas in the atmosphere, about 76% is in the form of N2 (a diatomic molecule, or one of the HONClBrIF twins). N Nitrogen molecules exist with a triple bond atom to atom, making it very stable. 7 2-5 It has the most oxidation states of all elements. When it makes an ion, it ONLY forms a -3 Anion. It gains 3 electrons to become ISOELECTRIC to neon. Nitrogen can make triple, double, and single bonds. The most important compound it makes is ammonia, which is NH3
15.9994 -2 Oxygen Without oxygen we would die in about 2 minutes, so it s important. It makes up about 18% of the air we live in. O Oxygen is one of the HONClBrIF twins, and it exists as a diatomic molecule of O2. 8 2-6 It can make double and single bonds. An obvious compound containing oxygen is water, the other is silicon dioxide which is beach sand. In compounds water is not diatomic. It can gain its stability by bonding to other atoms. It s always diatomic when in its pure state of just being oxygen. It ONLY makes a -2 anion, gaining 2 electrons to become ISOELECTRIC to neon.
18.9984 -1 Fluorine This gas is also a HONClBrIF twin, existing in its pure state as F2 molecules. F Fluorine is the most reactive nonmetal of all. It only makes a -1 anion, when it gains one electron to become ISOELECTRIC to neon. 9 2-7 It s in period 2, so it has two orbitals. It is in group 17, which are known as the HALOGENS. Sodium fluoride (NaF) is added to the water in most locales (Vestal included) and that ion is good for keeping your tooth enamel strong. Too much is not healthy. Some folks believe that adding fluoride to drinking water can control you, but that is a crazy conspiracy theory. Drink a lot of water.
20.180 0 Neon The second gas in group 18, the noble gases. Neon is inert as well. It makes no bonds, no ions, and has no interest in other atoms. It has TWO complete orbitals and is also perfect like helium, which is why it doesn t bond. Ne 10 2-8 Neon gas is used in open signs, and works well since when the 2-8 electron orbitals get excited, say to 2-7-1 which is unstable, when the excited electron returns to the ground state of 2-8, the energy released is visible as ORANGE light to your eyes. Many atoms that form ions become ISOELECTRIC to neon. That means they end up with the same 2-8 electron configuration as neon, but they do NOT become neon (that s crazy!). Metals lose electrons, nonmetals gain electrons to do this.
22.98977 +1 Sodium The second alkali metal in group 1, the first atom in period 3, meaning it s the first atom with three electron orbitals. The first two are full (2-8), the eleventh electron starts the third orbital. Na 11 2-8-1 Sodium makes ONLY a +1 cation, losing one electron to become ISOELECTRIC to neon. Sodium chloride is table salt, and wildly popular in oceans and your body too.
24.305 +2 Magnesium The second Alkaline Earth metal in group 2, the second atom in period 3, meaning it s the first atom with three electron orbitals. The first two are full (2-8), the third orbital has the 2 valence electrons. Those are the electrons it loses when it forms a +2 cation, and it becomes ISOELECTRIC to neon. Mg 12 2-8-2 You took magnesium and lit it aflame in lab, forming into magnesium oxide, MgO(S). Most metals, including magnesium, are malleable (means you can pound it flat), ductile (means you can draw it into a wire), and conduct both heat and electricity fairly well too. The atomic mass rounds to 24 amu, which means the most common isotope of magnesium is Mg-24.
26.98154 +3 Aluminum Atom 13 is also in group 13 (an odd coincidence). Aluminum has two full orbitals, and three valence electrons. It loses 3 electrons when it becomes a +3 cation, ISOELECTRIC to neon. Al 13 2-8-3 Aluminum is relatively soft, of low density, and strong. It does not corrode much, but can form a thin layer of aluminum oxide that does not get thicker. This leaves it sometimes less shiny, but as strong as ever. Very malleable so we make aluminum foil out of it, and airplanes too! It is in period 3, it has three orbitals. Aluminum does touch the staircase between the metals and nonmetals, but IT IS NOT A metalloid. Al and Po are the 2 exceptions to the metalloid rule.
28.0855 -4 +2 +4 Silicon In our class silicon does NOT make ions. It s a nonmetal and it s a Metalloid too. As a nonmetal it is brittle as expected, but strangely, it conducts electricity. Metalloids are sometimes called semi- metals because they have properties of both the nonmetals and the metals too. Si 14 2-8-4 Silicone is different that silicone, which is gel-like solid, sometimes used in cosmetic surgery. It contains silicon and other elements as well. Silicon is an ELEMENT. One of the most common substances on Earth is silicon dioxide, or beach sand.
30.97376 -3 +3 +5 Phosphorous Phosphorous is a solid (at STP) nonmetal. It ONLY makes a -3 anion, when it gains three electrons and becomes ISOELECTRIC to argon. P Phosphorous is in group 15, which makes it chemically similar to both nitrogen above, and arsenic below. 15 2-8-5 Chemical similarity means that these atoms all make similar bonds. All make -3 anions when making ionic bonds. All have three of the same oxidation states (-3, +3, and +5) so they will often bond covalently in the same proportions as well.
32.065 -2 +4 +6 Sulfur This is another solid nonmetal (at STP). It can only make a -2 anion when it bonds ionically. That is because it gains 2 electrons to become ISOELECTRIC to argon. S 16 2-8-6 Sulfur compounds are often smelly in a bad way. H2S is stink gas when you buy one of those bombs from what are called gift shops at the mall. Rotten eggs smell so badly because of that gas as well. Solid, elemental sulfur is yellow and hard, like a rock. It can be ground up into a powder too, but does not smell very much at all.
35.453 -1 +1 +5 +7 Chlorine Another HONClBrIF twin, it exists as a diatomic molecule when pure. Chlorine gas is greenish in color and smells a lot like death. If you breath it, this gas will cause severe chemical burns in your mucus membranes and lungs, it will be terrible. Cl 17 2-8-7 People who put chlorine into their pools are just not good chemists. They put in a white crystalline ionic compound called sodium hypochlorite (NaClO) but call it by a casual name. Chlorine will kill you, but when this bonds ionically with sodium, making table salt, it s required by living organisms for survival. Chlorine ONLY makes a -1 anion when it gains one electron to become ISOELECTRIC to argon. Chlorine in group 17 is a HALOGEN.
39.948 0 Argon The third noble gas, equally inert to the two above it in the group (He and Ne). It has three full orbitals and that makes it perfect and not willing to bond with other atoms. Ar 18 2-8-8 All noble gases have ONLY full orbitals. Each noble gas exhibits full electron orbitals only. Strangely (since these atoms never expected YOU to learn about them, this third orbital can be full with eight electrons, or it can stretch out and fill up and hold 18 electrons (look below at krypton). It s the last atom in period 3, it s the last atom with just 3electron orbitals.
39.0983 +1 Potassium This is the third Alkali Metal in group 1, and as the two atoms above it (Li and Na) it has just one valence electron. This metal will ONLY make a +1 cation, it bonds similarly to both Lithium and Sodium when making ionic bonds. K 19 2-8-8-1 Potassium becomes ISOELECTRIC to argon. Each step lower in the group makes the metals similar, but more reactive. Sodium is the first atom with four electrons orbitals, it s the first atom in Period 4. The atoms of period 4 range from reactive metals, less reactive metals, metalloids, nonmetals and finally a noble gas. Their ONLY similarity is that they have four electron orbitals. Atoms in groups are similar chemically.
40.08 +2 Calcium This is the third atom in group 2, the Alkali Earth Metals. It s more reactive than either beryllium or magnesium. It has the obvious similarity of having two valence electrons. It LOSES ONLY two valence electrons when forming a +2 cation. When it s an ion, it is ISOELECTRIC to argon. Ca 20 2-8-8-2 Calcium in in your bones and also sea shells. When you drink milk that contains calcium , you realize that the milk has no metal in it. Rather, you re drinking calcium cations in solution.
44.9559 +3 Scandium This is the first of the transitional metals, the elements in the central block of the periodic table from group 3 through 12. Sc Scandium is the first atom that does not follow the simple ISOELECTRIC rule, as the atoms mid-table usually can make more than one cation. 21 2-8-9-2 That s possible when you learn more chemistry, and about the sub-orbitals that the electrons really exist in. Regents chem is an intro course, and you learn about orbitals, but it is a bit more complex in real life. Since there is a +3 in the top right corner, you now know that scandium ONLY makes a +3 cation, but not really why. That s okay, a +3 cation, let s move on from here.
47.867 +2 +3 +4 Titanium This is the first truly exotic atom in the transitional groups. Titanium has the ability to lose 2 electrons and be a stable +2 cation; or it can lose 3 electrons, forming a stable +3 cation; or, it can lose 4 electrons, forming a stable +4 cation. Ti 22 2-8-10-2 Since all atoms have their electrons in sub-orbitals of the orbitals, and you don t really know about them, this atom can make three different cations, depending on how many electrons it transfers. We would call these cations by the names of titanium II, or titanium III, or titanium III. The roman numeral matches the charge of the cation. If a metal can make more than one cation, you MUST USE a roman numeral in it s name, but not in it s formula.
50.9415 +2 +3 +4 +5 Vanadium This metal can make four different cations, all shown top right in the periodic table box. V When vanadium and chlorine combine to form vanadium chloride, you must clearly indicate what type of vanadium chloride you are talking about. Each type, from each vanadium cation has a different formula: 23 2-8-11-2 VCl2 is vanadium (II) chloride VCl3 is vanadium (III) chloride VCl4 is vanadium (IV) chloride VCl5 is vanadium (V) chloride Note: NO ROMAN NUMERALS in the formulas, only in the word names! Without a roman numeral name, all four of these different compounds would be vanadium chloride, which means we d all get mixed up, which we won t let happen.
55.996 +2 +3 +6 Chromium This metal only makes 3 different cations, the last one is a carcinogen, which means it can cause cancer in humans. Cr Chromium (II) sulfide is CrS 24 2-8-13-1 Chromium (III) sulfide is Cr2S3 Chromium (VI) sulfide is trickier, you might think it s Cr2S6, but John Dalton would say SIMPLE WHOLE NUMBER RATIOS PLEASE: so it really is CrS3.
54.9380 +2 +3 +4 +7 Manganese Manganese can make a +7 cation, which seems odd. It is, and the reason for this (in our class) is that it can and you don t need to understand why. You just look in the top right hand corner and the positive numbers tell you what cations are possible. Mn 25 2-8-13-2 Cation charges can go from +1 through +7. Roman numerals from 1 to 7 look like this: I II III IV V VI VII To tell time you might need to count to 12. To count that high, use these for 8 through twelve: VIII IX X XI XII
55.845 +2 +3 Iron A common metal on Earth and in the Universe. Iron is in period 4, it has FOUR electron orbitals. It forms two kinds of cations, +2 and +3. Fe The +3 cation combined with oxygen makes rust. Rust is weaker than iron, and that s is one really good reason to have invented paint. 26 2-8-14-2 Paint is a mostly water tight seal between the iron (or steel alloy) inside, and the air and rain on the outside. The rain or salty water makes the rusting reaction happen even quicker. Iron is in group 8, period 4. It is the ONLY atom in the Universe with 26 protons. Iron has 4 stable isotopes and 24 radioactive ones. The radioactive ones break down with chemistry we still have to learn about.
58.9332 +2 +3 Cobalt Cobalt is another transitional metal, and like iron makes the same two kinds of cations, +2 and +3. Co Stable cobalt is used for making a variety of cobalt blue pigments when cobalt is bonded ionically. 27 2-8-15-2 The isotope Co-60 emits beta particles and is used by doctors who shoot these radioactive particles at hard to remove tumors and cancers. The radioactive particles are able to transmit through the body and be aimed at the bad cells inside a person, hopefully killing them off. It s third orbital is stretching out a bit, remember it fill up with 8 electrons, but can stretch out to hold up to 18 electrons. That s due to all of the (mildly) complex sub-orbitals that we don t cover in high school chemistry class.
58.693 +2 +3 Nickel This is the third element in a row that has the same two kinds of cations. Kids often spell this atom as nickle, as in pickle, but that s wrong! Ni If I had a nickel for every time I saw nickle, I d be rich by now! 28 2-8-16-2 Nickel is in our nickel coins, but those are really only 25% nickel, and 75% copper. When metals are melted together (mixed) and then allowed to cool back to a solid, it s called forming an ALLOY. Alloys are usually stronger or have a quality that the metals alone do not possess. Nickel coins are remarkably strong and durable. And inexpensive, and don t look like big pennies either. Again, it makes that same two cations, +2 and +3.
63.546 +1 +2 Copper Most metals are silver colored, some shinier, some duller. Copper is reddish and way cool. Cu Copper is common, and we use it for wires because it is very ductile, has low resistance to electricity, and is so pretty. Well, the pretty part is not really why we use it this way. 29 2-8-18-1 Copper can be melted with tin to form brass, another alloy that is used for the brass instruments like trumpets and tubas. Copper makes different cations, +1 and +2 only. A member of period 4, it has four electron orbitals. Each orbital is also an energy level. When copper and copper compounds are excited, they emit green color light as spectra.
65.409 +2 Zinc Zinc is the first transitional metal since number 21 (scandium) to make only one kind of cation, +2. The reason for this is that it s sub-orbitals more closely match the metals in group two, and that even though there are sub-orbitals, they are very close to the alkaline earth metals. Zn 30 2-8-18-2 No roman numerals are allowed for zinc. Since it makes ONLY ONE cation using a roman numeral is sort of overdoing things. You would never use a roman numeral for table salt, like this: sodium (I) chloride. Sodium can ONLY makes that +1 cation, you would not need to say it twice. Zinc chloride is ZnCl2, because zinc makes a +2 cation. Everyone even a little be bright already knows that, the roman numeral is not needed here.
69.723 +3 Gallium Gallium is number 31 (31 p+1nd 31 e-1 as well. It s underneath aluminum, atom number 13, in the 13th group. Another odd coincidence, the reverse of 13 is 31, both atoms are in group 13. Ga 31 2-8-18-3 Gallium only makes a +3 cation, no roman numerals are used ever. Gallium has a very low melting point, and was used as a Victorian England gag. Come over to someone s house for a spot of tea. Get a spoon that looks silver (how high class!) but when you stir your Earl Grey tea, the spoon disappears. You think something awful has happened, but your (smart-aleck) friends laugh off their heads, metaphorically speaking.
72.64 +2 +4 Germanium This is another nonmetal but also a metalloid. In our class we will not likely see it, but it could make a +2 or +4 cation. Roman numerals are necessary. Ge A metalloid can be a nonmetal with some metallic properties (like this element), or like arsenic (the next atom coming) which is a nonmetal with some metallic properties. 32 2-8-18-4 Metalloids are sometimes known as semi-metals because they have some metallic properties, but not as strongly exhibited as with standard metals. There are only 7 metalloids, even though 9 atoms touch the staircase. Al and Po are metals, and exceptions to this rule . The metalloids include B, Si, Ge, As, Sb, Te, and At. Metalloids sounds like the name to a punk band from Great Britain.
74.9216 -3 +3 +5 Arsenic The first nonmetal in period 4, arsenic is also a metalloid. In our class arsenic will not make ions, but it has three oxidation numbers. It will bond like nitrogen and phosphorous in that it shares three of the same oxidation states. As 33 2-8-18-5 Arsenic can poison humans. Small doses can induce confusion, headaches and diahrrea. Excessive doses can cause coma and death.
78.96 -2 +4 +6 Selenium The third atom in group 16, selenium bonds similarly to both oxygen and sulfur by making ONLY -3 anions. It gains 3 electrons to become ISOELECTRIC to krypton. Se 34 2-8-18-6 Selenium has six naturally occuring isotopes but more than 20 more have been isolated in nuclear reactions. Small amounts you get in foods keep you healthy (200 micrograms per day). Double that and you overdose, so don t take selenium supplements (or else!). It s a solid nonmetal, but is mostly found in compounds and not in it s elemental form.
79.904 -1 +1 +5 Bromine This is the third HALOGEN of group 17. It makes ONLY a -1 anion, gaining one electron to become ISOELECTRIC to krypton. Br Bromine is another of the diatomic elements, a HONClBrIF twin, existing in it s pure form as Br2. Bromine has a melting point of 266 K and doesn t boil until 332 K. Since room temperature is about 298 Kelvin, bromine is naturally a liquid at normal temperature for humans. 35 2-8-18-7 Bromine is not necessary for human metabolism, and has limited uses for us, other than in some chemical reactions, photography, etc.
83.798 0 Krypton +2 The fourth noble gas. This one has four complete electron orbitals, with that third orbital stretched out to the max with 18 electrons. Kr Noble gases were once thought to be inert, completely unreactive. Krypton (and also xenon) have been forced to make some compounds under some extreme conditions. The first time a noble gas was chemically combined by scientists happened in 1962, when KrF2, or krypton difluoride formed under high pressure and temperature. This is not normal and like most noble gases, it tends to not make compounds. 36 2-8-18-8 Krypton gas lamps are used in signs, but is relatively rare in the atmosphere. It s very unhealthy to breathe this gas, but even finding some is difficult, no worries.
85.4678 +1 Rubidium The fourth Alkali Metal of group 1, with the characteristic single valence electron. Rubidium, like all group 1 metals ONLY makes a +1 cation when making ionic bonds. Rb 37 2-8-18-8-1 Rubidium is more reactive than potassium and less so than cesium, the metal below it. Rubidium has 37 electrons (and 37 protons). The electron configuration clearly shows the start of the new 5th orbital, necessary since the first four orbitals are full up, there is no room for that 37th electron without the fifth orbital.
87.62 +2 Strontium This is the fourth Alkaline Earth Metal, with the expected 2 valence electrons. Strontium ONLY makes a +2 cation when making ionic bonds. No roman numerals are necessary (or allowed). Sr 38 2-8-18-8-2 Radioactive isotopes of strontium give off dangerous radioactive particles which can kill humans. The problem with these is that strontium is chemically similar to calcium, and if you somehow ingest radioactive strontium, it can bond into your bones (as does calcium), locking the radioactive isotopes in you (that s bad).
88.9059 +2 Yttrium The first element with a truly weird name, and a symbol that looks like the start of a Village People song (YMCA!). Y Yttrium is a transitional metal, but just like scandium right above it, yttrium ONLY makes a +3 cation when it bonds ionically. 39 2-8-18-9-2
91.224 +4 Zirconium This element makes only a +4 cation (kind of cool). Zr It s in period 5, so as expected it has five electron orbitals. With 40 electrons, it has 40 protons too. 40 2-8-18-10-2 Since the total mass (rounded) is 91 amu, the most common isotope of zirconium has this mass. We also know 40 of these amu are from the protons, meaning zirconium has 51 neutrons.
92.9064 +3 +5 Niobium This is a really cool name for a neat metal. It s transitional, so it can make 2 different cations, Those being a +3 and a +5 cation. That s possible because of the sub-orbitals that we don t have to know much about in high school level chemistry. Nb 41 2-8-18-12-1 Niobium is in group 5, period 5.
95.94 +6 Molybdenum Hard name, cool metal. Say mull-ib-da-num . Mo This metal ONLY makes a +6 cation, which is pretty cool. Just above it is chromium, which also can make this same +6 cation. 42 2-8-18-13-1 This metal can be used to form an alloy with iron, making a super strong super-alloy . Strangely, it has 35 known isotopes (!).
(98) +4 +6 +7 Technetium One of the oddest of all, this is the lightest elements that all of the isotopes are radioactive. That means none of this is stable, it undergoes radioactive decay and transmutes into other elements (we ll learn about that later in the year). Tc 43 2-8-18-13-2 Note it s mass is in parenthesis, and shows just 98 , which means that the mass is difficult to measure more accurately. We will not see this in high school chem, nor will you have to make compounds with it. It s real, but pretty funky for our chemistry.
101.07 +3 Ruthenium A non-reactive silvery colored metal (like platinum) and very unreactive (like platinum and other precious metals like gold and silver, etc.). Ru It s name comes from an old fashioned name for Russia. It s mined there, but not found in a pure form, it has to be decomposed from ionic compounds. 44 2-8-18-15-1 That cation is more for us to play with on paper, but less likely to form in real life.
102.906 +3 Rhodium A non-reactive silvery colored metal (like platinum) and very unreactive as well. Rh It s name comes from the color (rose) of the precipitate that it was first discovered in. 45 2-8-18-16-1 This is not a common element for high school chemists. It is in period 5, it has five electron orbitals, and it has one valence electron. That +3 cation forms because orbital stability of the sub-orbitals that we don t use in our class allows it to. It does not form ions often (which means it s unreactive). That cation is more for us to play with on paper, but less likely to form in real life.
106.42 +2 +4 Palladium Another precious metal, mostly used in vehicle catalytic convertors , which convert toxic fumes into less dangerous gases. Pd The strangest thing to note is that it has only 4 electron orbitals even though it is in period 5! 46 2-8-18-18 This element has five full d suborbitals, which allows this exception to happen. Palladium does not react at all with oxygen, so it does not tarnish. It makes nice jewelry that stays shiny forever, which is why it s so valuable. A nice ring made of iron would rust, which makes a metal like that (more reactive with oxygen) less valuable. Two cations (two roman numerals) but these are more for thinking in class, it s not likely to make ions and bond to anything in real life.
107.868 +1 Silver Another precious metal, often used in jewelry because it does not tarnish much. It is also been part of the money systems throughout history (along with gold). Ag 47 2-8-18-18-1 Silver is near the bottom of the Reactivity Series (table J) because it has such low reactivity. It s very shiny, very ductile, and makes many different kinds of alloys. Silver makes ONLY a +1 cation
112.41 +2 Cadmium Like zinc above it in group 12, it makes ONLY a +2 cation. Cadmium is not needed for human metabolism and has uses in batteries. Cd It s used less so as it is being recognized as toxic to humans by scientists. 48 2-8-18-18-2
114.818 +3 Indium Like the two metals above it in group 13 (aluminum and gallium) this metal also ONLY makes a +3 cation when it forms ionic bonds. It s in period 5 with the expected five electron orbitals. In 49 2-8-18-18-3 This is a relatively soft metal (like many nearby it in the table (Al, Ga, Sn, Pb). It is not part of human metabolism.