Free Radical Substitution Mechanism in Methane Chlorination
Explore the detailed steps of the free radical substitution mechanism in the chlorination of methane, including chain initiation, propagation, and termination. Learn about the role of common molecules like O2 in slowing or stopping the reaction and discover how radical-radical recombination reactions influence the overall process. Dive into the nuances of the reaction and understand factors like temperature, light input, and reactivity rates of different halogens.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
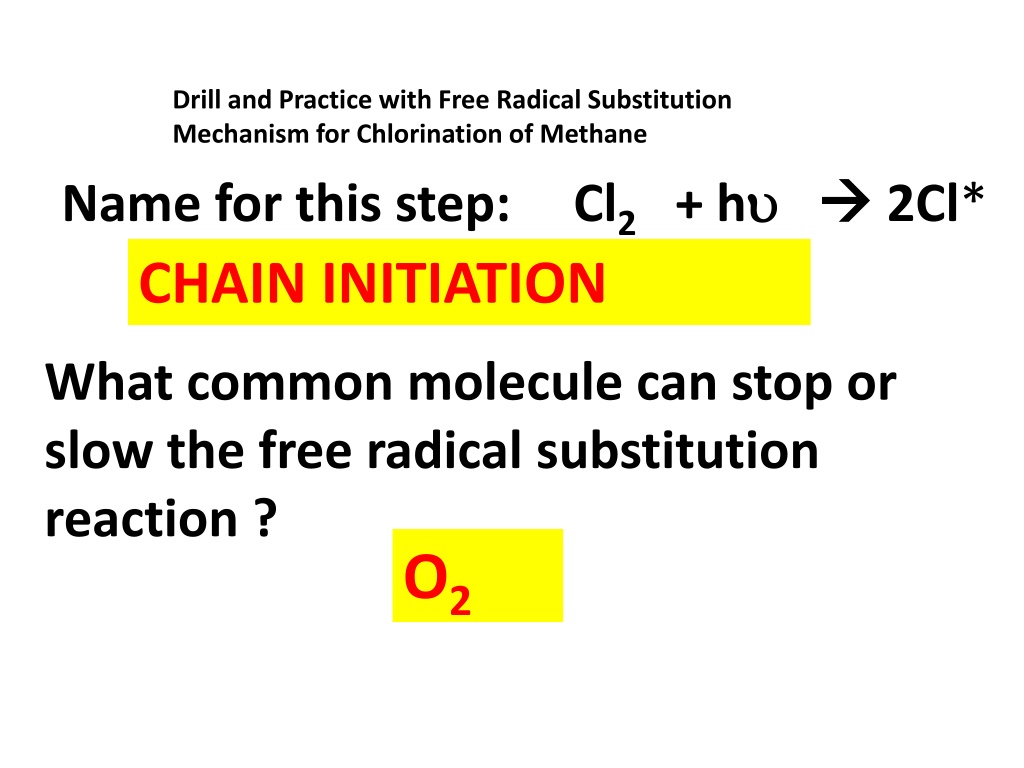
Drill and Practice with Free Radical Substitution Mechanism for Chlorination of Methane Name for this step: Cl2 + h CHAIN INITIATION 2Cl* What common molecule can stop or slow the free radical substitution reaction ? O2
Drill and Practice with Free Radical Substitution Mechanism for Chlorination of Methane (continued) What is the second step of the methane chlorination mechanism ? Step 2) Cl* + CH4 HCl + CH3* (radical) What is the name of the step(s) that rationalize the high photoyield of the methane chlorination reaction ? CHAIN PROPAGATION
Drill and Practice with Free Radical Substitution Mechanism for Chlorination of Methane (continued) Radical-radical recombination reactions have what effect on the radical chlorination reaction of methane ? TERMINATES THE CHAIN REACTION http://vectors.umwblogs.org/files/2012/02/Movies_Films_T_The_Terminator_010629_-12.jpg What step follows: Step 2) Cl* + CH4 STEP 3) CH3* + Cl2 CH3* + HCl CH3Cl + Cl*
Drill and Practice with Free Radical Substitution Mechanism for Chlorination of Methane (continued) Which of the 5 facts is missing ?? reaction will only go at >250oC in dark or with input of ultraviolet (uv) light. in xs Cl2 , a single photon (h ) event causes thousands of halogenations. O2 causes halogenation reaction to slow or stop. Reactivity rate follows: F2 > Cl2 > Br2 >I2 Wavelength of uv light that initiates reaction is the decomposition wavelength for the halogen
Drill and Practice with Free Radical Substitution Mechanism for Chlorination of Methane (continued) Which is not a step in the radical chlorination mechanism of methane?: a) Cl* +CH4 b) Cl* +Cl* c) CH3 + HCl d) Cl2 + CH3* CH3* + HCl Cl2 CH3Cl + H* CH3Cl + Cl* c) CH3 + HCl CH3Cl + H*
Drill and Practice with Free Radical Substitution Mechanism for Chlorination of Methane (continued) Chain termination, chain initiation and chain _______________ propagation What reaction explains the quenching effect of O2 on the radical substitution of Cl on methane ?? CH3* + O2 methylperoxy radical (~ stable) CH3OO *
Drill and Practice with Free Radical Substitution Mechanism for Chlorination of Methane (continued) Activation barrier/energy energy Step 2: CH4 + Cl* What s this barrier/energy called ? What reaction step and reaction here ? Time or reaction progress
Drill and Practice with Free Radical Substitution Mechanism for Chlorination of Methane (continued) `activated complex What does the Xmas seal sign stand for ? energy HX + CH3* What species lives here? Time or reaction progress
Drill and Practice with Free Radical Substitution Mechanism for Chlorination of Methane (continued) What reaction step and reaction here ? energy Step 3: Cl2 +CH3 Name of postulate connected to activated complex picture Hammond Postulate CH3Cl + Cl* Time or reaction progress
ANY DAY DOING ORGANIC CHEMISTRY IS A ____________DAY GOOD https://encrypted-tbn0.gstatic.com/images?q=tbn:ANd9GcQbiMmW4cYC41y_S-NF4x27HGmMwmYVOiZE0D6pz3W_7eGLAKCgkg