The Impact of J.J. Thomson's Discovery of the Electron on Atomic Theory
J.J. Thomson's groundbreaking discovery of the electron in 1897 revolutionized the understanding of atoms. His Plum Pudding Model proposed in 1904 depicted electrons embedded in a positively charged matter, challenging the previous Dalton's Billiard Ball Model. This discovery transformed the atomic model, revealing atoms are composed of negatively charged particles known as electrons, leading to a more detailed structure explanation.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
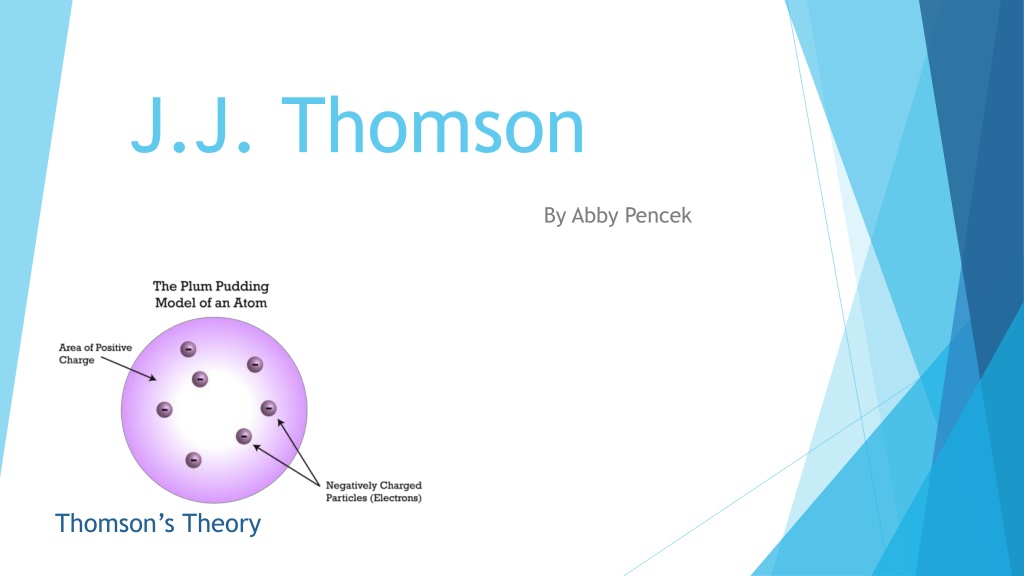
J.J. Thomson By Abby Pencek Thomson s Theory
Background info of Thomson Born: December 18, 1856 Real name: Joseph John Thomson Died: August 30, 1940 Discovered: Electron When: 1897 Experiment: Cathode Ray Tube Theory: Plum Pudding Model in 1904 Won a noble prize
Discovery in 1897 Thompson experimented with cathode ray tubes and discovered the electron The cathode ray produced radiation when a voltage is [joined] between two metal plates inside a glass tube that is filled with low pressured gas that consists of particles, or electrons, that conduct electricity (NoblePrize). Proposed in 1904 the plum pudding model of an atom Electrons surrounded in a positively charged matter
How J.J. Thomson changed the understanding of an atom Before this discovery, the only known thing was an atom. Then, Thomson found that atoms are made up of negatively charged particles called electrons. Therefore, the atomic model, became more detailed.
Before the discovery Dalton s Billiard Ball Model was used before Thomson s discovery and model was created. Dalton s Billiard Ball was the first model of a atom. Thomson added electrons surrounded in a positively charged pudding to the model.
The Discovery and Experiment Video Discovery of the Electron: Cathode Ray Tube Experiment- 11:07 https://www.youtube.com/watch?v=Rb6MguN0Uj4 J.J. Thomson s CTR Experiment- The Discovery of the Electron- 10:43 https://www.youtube.com/watch?v=DIC9uE_p2e4
Work Cited Discovery of the Electron and Nucleus. Khan Academy, Khan Academy, www.khanacademy.org/science/chemistry/electronic-structure-of- atoms/history-of-atomic-structure/a/discovery-of-the-electron-and-nucleus. J.J. Thomson - Biographical. Nobelprize.org, www.nobelprize.org/prizes/physics/1906/thomson/biographical/. J.J. Thomson. Biography.com, A&E Networks Television, 20 Sept. 2017, www.biography.com/people/jj-thomson-40039. J.J. Thomson Facts. NobelPrize.org. Nobel Media AB 2018. Thu. 16 Aug 2018. https://www.nobelprize.org/prizes/physics/1906/thomson/facts/. John Dalton. The History of the Atom, thehistoryoftheatom.weebly.com/john- dalton.html.