Understanding Fossil Fuels and Pollution: A Comprehensive Overview
Fossil fuels such as coal, oil, and natural gas are non-renewable energy sources that release heat energy when burned, but also contribute to pollution when not burned completely. This leads to the release of harmful pollutants like carbon dioxide, carbon monoxide, sulfur dioxide, and carbon particulates into the atmosphere. Complete combustion with sufficient oxygen produces carbon dioxide and water, while incomplete combustion with insufficient oxygen can lead to the formation of carbon monoxide and soot. These pollutants have detrimental effects on the environment, human health, and contribute to global warming.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
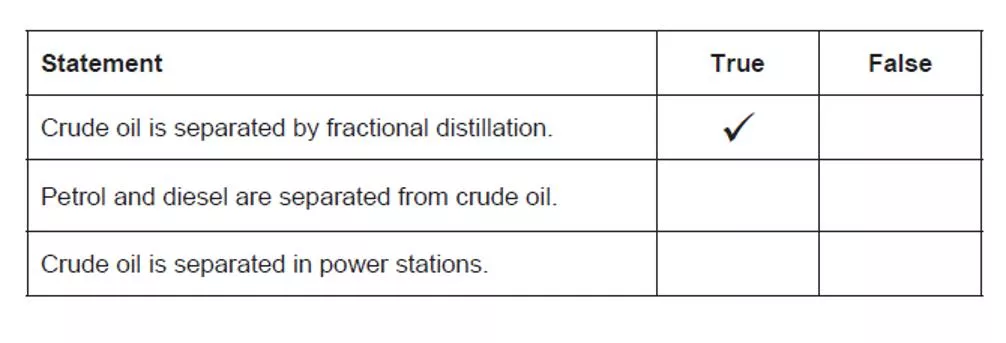
Fossil fuels and pollution Which statements are true? Stretch: Why are fossil fuels such as coal, oil and gas classed non-renewable?
Where is crude oil separated? The refinery- during a process called fractional distillation
What is released into the atmosphere when fossil fuels are burned?
Fossil fuels Fossil fuels include coal, oil and natural gas. They were formed from the remains of living organisms millions of years ago and they release heat energy when they are burned. They are non- renewable. They have chemical energy stored within them. About three-quarters of the electricity generated in the UK comes from power stations fuelled by fossil fuels
Most fuels contain carbon and/or hydrogen. Some also contain Sulphur. When these fuels are burned in a combustion reaction atmospheric pollutants are formed. Complete combustion Complete combustion of a hydrocarbon fuel occurs when there is a good supply of oxygen. When fuels contain Sulphur- Sulphur dioxide is produced. It releases the maximum amount of energy and produces carbon dioxide and water. Methane + Oxygen Carbon dioxide + Water Fuel Complete combustion Plenty of Oxygen
Carbon monoxide is a deadly gas why? Incomplete combustion Incomplete combustion of a hydrocarbon fuel occurs when there is a poor supply of oxygen. Less energy is released. Water is still produced from the hydrogen atoms. Instead of carbon dioxide, you might get carbon monoxide or particulate carbon, known commonly as soot, or a mixture of both. Ethane + Oxygen Carbon Monoxide + Water Or Carbon particulates Fuel Not much Oxygen Incomplete combustion
The pollutants: Carbon dioxide- is very bad for the environment in large amounts. Increased cardbon dioxide leads to global warming. Carbon monoxide- is a very dangerous gas that can cause death. It is colourless, odourless and VERY VERY toxic/poisonous. Carbon particulates- are soot. They cause global dimming, causing thick smog (smoke and fog). This is harmful for the human lungs. Sulphur dioxide- causes acid rain, which can damage environments and can be bad for human health.
What is the difference between complete and incomplete combustion? Use the keywords below to fill in the gaps. In complete combustion there is lots of __________ present when the fuel is burned. ___________ __________ is produced during complete combustion. Carbon dioxide causes global __________. During incomplete combustion ________ __________ is produced because there is not as much oxygen produced. __________ particulates can also be produced in incomplete combustion, this causes global _________. KEYWORDS: Carbon monoxide, dimming, warming, Carbon dioxide, Oxygen, Carbon. Carbon Monoxide is a deadly gas because ________________________________________________________________ ________________________________________________________________ __________ Burning fuels that contain Sulphur and nitrogen that can lead to __________ __________. Which can be harmful to the environment and to human health.
What gases are produced when burning fossil fuels? How is complete combustion different to incomplete combustion?