Insights into Solvation and Polarizability Effects on pKa Values in Organic Chemistry
Learn about how solvation effects and polarizability impact the pKa values of charged molecules in organic chemistry. Solvents can stabilize charges, while polarizability influences the spread of charge in molecules. Discover how conjugate base and acid stabilization play key roles in determining pKa values, as explained through examples and trends observed in various substrates.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
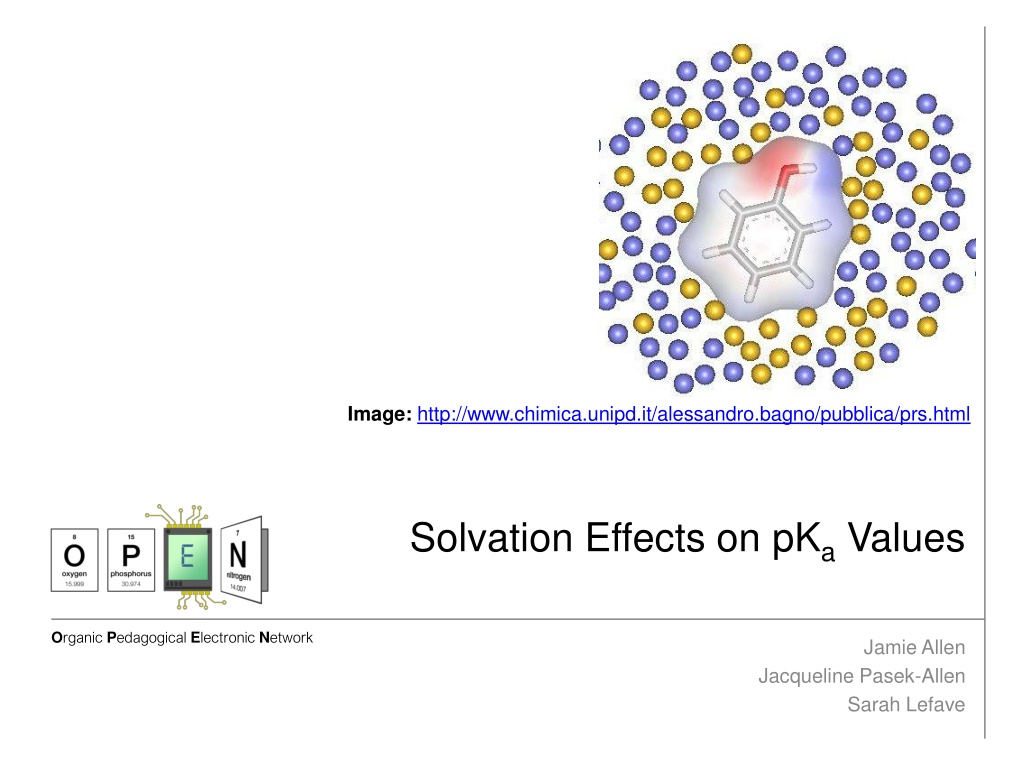
Image: http://www.chimica.unipd.it/alessandro.bagno/pubblica/prs.html Solvation Effects on pKaValues Organic Pedagogical Electronic Network Jamie Allen Jacqueline Pasek-Allen Sarah Lefave
Solvation effects on charged molecules When substrates are put in solution, the solvent molecules can organize themselves around a charged species to stabilize it. Solvents can stabilize a charge most effectively when the charge on the substrate is easy to get to. That being said, sterically hindered ions are harder for solvents to stabilize then non-sterically hindered molecules. Conjugate base stabilization pKa = 16 R-AH-> R-A- + H+ neutral -> negative Stabilization of the conjugate base leads to a lower pKa. When addressing conjugate base anions, the stronger acids are seen in less sterically-hindered molecules. This is because more solvent molecules can surround the negative charge, thus higher conjugate base stabilization drive the reaction to the right R group size = pKa pKa = 18 Acid stabilization pKa =10.62 R-A+H-> R-A +H+ positive -> neutral Stabilization of the acid leads to a higher pKa. When addressing acid cations, the stronger acids are seen in more sterically hindered molecules. This is because less solvent molecules can surround the positive charge, thus lower acid stabilization drives the reaction to the left. R group size = pKa pKa =9.76 pKa table: http://www.3rd1000.com/chem301/p00405.htm Other References: Anslyn, Eric V., and Dennis A. Dougherty. Modern Physical Organic Chemistry. Sausalito, CA: U Science, 2006. Print.
Polarizability effects on pKa A molecule is stabilized when it can spread out it s charge over the greatest amount of area. When predicting pKas based on polarizability effects, one measures the amount of charge that the anion/cation itself can spread out via its orbital size and substituents. Pure polarization effects are seen in the ideal gas phase where solvent (therefore, solvation effects) are absent. However, if polarization effects are strong enough, they can be seen in the presence of solvent as well. Conjugate base stabilization pKa = 3 R-AH-> R-A- + H+ neutral -> negative pKa = -8 pKa = -9 When comparing halogens, iodine has the lowest pKa because the conjugate base s anion is able to spread out the most charge through iodine s large size. orbital size = pKa pKa = -10 Acid stabilization R-A+H-> R-A +H+ positive -> neutral pKa = 11 Triethylammonium is more stable than ammonium because the alkyl groups aid in spreading out the positive charge. Thus, triethylammonium has the highest pKa because the acid is stabilized. substituent size = pKa pKa = 9 References: http://employees.csbsju.edu/cschaller/Principles%20Chem/acidity/acid%20local.htm
The curious case of Ammonium ion derivatives pKa trend based on a mixture of polarizability and solvation effects Some substrates can exhibit a mixture of both polarization effects and solvation effects, thus creating a unique trend in acidity. An example is shown in the following pKa trends of ammonium derivatives compared to predicted trends. Predicted pKa Trends: Experimental pKa Trends: Lowest pKa Polarizability Highest pKa Polarizability predicts (CH3)3NH+ as the weakest acid because more alkyl groups spread out the positive charge better. Lowest pKa Highest pKa ACTUAL Actual pKa values indicate that both polarizability and solvation effects act in concert in this case, with (CH3)3NH+in the middle of the pKa trend. Lowest pKa Solvation Highest pKa Solvation predicts (CH3)3NH+ as the strongest acid because it is the hardest for the solvent to get to and stabilize the positive charge. References: Anslyn, Eric V., and Dennis A. Dougherty. Modern Physical Organic Chemistry. Sausalito, CA: U Science, 2006. Print.
Case Study 1: Experimental Solvation Effects on Relative Acidity of Alcohols In the 1950 s the following order of acidity was observed in solution: CH3OH > C2H5OH > t-C4H9OH In the gas phase,the opposite acidity trend is seen: t-C4H9OH > C2H5OH > CH3OH In 1973, the above qualitative order was quantified with the following data in the gas phase: CH3O- + C2H5OH C2H5O- + CH3OH K1 = 23 2 K >> 1 indicates that the reaction lies heavily toward to the products and therefore indicates ethanol is a stronger acid than methanol. Addition of a solvent-like particle (CH3O- H OCH3) was done in the gas phase to study the effects on acidity with a very small solvation effect: CH3O- H OCH3 + C2H5OH C2H5O- H OCH3 + CH3OH K2 = 7.5 2 The above figure visually shows the effects of adding a solvent-like particle to a mixture of ethanol and methanol. Conclusion:The above reaction and equilibrium constant emphasize the effects that even one solvent-like particle can begin to switch the relative acidity of methanol vs. ethanol in the gas-phase. References: McIver, R.T.; Scott, J.A.; Riveros, J.M. Effect of Sovation on the intrinsic elative acidity of methanol and ethanol. J. Am. Chem. Soc., 1973, 95 (8), pp 2706 2708
Case Study 2: A modern approach to pKa estimates: Absolute pKa Determinations of Substituted Phenols Summary: As previously discussed, one simple method, such as solvation or polarization, rarely can accurately describe the pKa trends of acids. In the more modern times, a computational approach using continuum CPCM solvation calculations can been used. Specifically this method uses computations that combine both electronic and steric (polarization and solvation) effects, and chemists have used it to accurately predict the pKas in the solvent-phase of phenol derivative structures. Below are 2 trends that have been shown, along with their computationally predicted and experimental pKa values respectively. < < < 7.66 8.56 7.49 7.57 9.88 9.98 9.97 10.08 Predicted pKas: Experimental pKas: < < < Predicted pKas: Experimental pKas: 9.84 9.38 10.45 10.21 10.80 10.30 9.88 9.98 Note: It still holds that this method is not accurate for pKa predictions of gas-phase phenol derivative structures as they deviate significantly from those in solvent phase. References: Liptak, M.D.; Gross, K.C.; Seybold, P.G.; Feldgus, S.; Shields, G.C. Absolute pKa Determinations for Substituted Phenols. J. Am. Chem. Soc., 2002, 124 (22), pp 6421 6427
Questions 1. Would the following molecule be more stable in the gas phase or in a polar solvent solution? 5. If only solvent effects were at play in the following pKa trend, what order would the following phenols be in, in order of increasing pKa. < < < 4 2 3 1 a) Polar Solvent b) Gas-phase c) Same d)Neither 2. Is the following trend because of polarization effects, solvation effects, neither, or both? 9.88 9.98 9.97 10.08 7.66 8.56 7.49 7.57 a) 1, 2, 3, 4 b) 4, 3, 2, 1 c) 2, 1, 3, 4 d) 3, 2, 4, 1 Answers 1) b) In the gas phase; the many alkyl chains are able to spread out the positive charge. A polar solvent would be unfavorable because solvation effects would cause it to be unstable. 2) c) Neither. This trend follows the solvation trend; however, the effect is mainly due to induction. 3) d)the size and amount of halogen and R groups increases polarization and pKa of the molecule 4) b) decrease the original equation in the gas phase shows that p-ethylphenoxide is much more reactive than phenoxide, upon addition of a solvent-like molecule the K will decrease indicating that the relative acidity of the p-ethylphenoxide compared to phenoxide is decreasing. 5) d) the expected trend with solvation effects will have the least sterically hindered alcohol with the lowest pKa and the most hindered alcohol with the highest pKa. a) Polarization b) Solvation c) Neither d) Both 3. What is the order of pKalowest to highest due to only polarization effects? 1 2 3 4 a) 1, 2, 3, 4 b) 2, 1, 3, 4 c) 3, 1, 2, 4 d) 3, 4, 2, 1 4. How would you expect the K of the following reaction to change upon addition of C6H5O- H OCH3 K >> 1 + + a) Increase b) Decrease c) Same d) Cannot predict
Contributed by: Jamie Allen, Jacqueline Pasek-Allen, Sarah Lefave (Undergraduates) University of Utah, 2016 This work is licensed under a Creative Commons Attribution- ShareAlike 4.0 International License.