Understanding Fluconazole: Mechanism, Spectrum, and Clinical Applications
Explore the mechanism of action, spectrum of activity, pharmacokinetics, and clinical uses of fluconazole. Learn about its role in treating various fungal infections, including candidiasis, cryptococcosis, and dermatophytosis, while being aware of resistance issues and dosing considerations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
FLUCONAZOLE Pascalis Vergidis, MD, MSc Infectious Diseases Consultant Manchester University NHS Foundation Trust
Learning Objectives To understand the mechanism of action of fluconazole To be aware of fluconazole resistance and cross resistance to other antifungal drugs To be familiar with the pharmacokinetics and pharmacodynamics of fluconazole To be aware of the various indications of fluconazole prophylaxis and therapy To be aware of the different doses of fluconazole and their use in adults and children To know adverse reactions related to fluconazole
Structure Fluconazole is a first-generation synthetic triazole Molecular weight: 306.3 g/mol
Structure Voriconazole and fluconazole are structurally similar
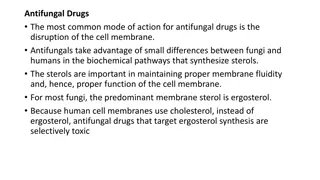
Mechanism of Action Preferentially binds fungalcytochrome P450-dependent lanosterol C- 14- demethylase Inhibits the conversion of lanosterol to ergosterol major constituent of fungal cell membrane Resulting in the accumulation of fungal 14 alpha-methyl sterols, the loss of normal fungal sterols, and fungistatic activity. Mammalian cell demethylation is much less sensitive to fluconazole inhibition. Fungicidal to Cryptococcus spp in a dose-dependent pattern
Mechanism of Action Acetyl-CoA Squalene Squalene monooxygenase Squalene -2,3 oxide Lanosterol C-14 demethylase Azoles Ergosterol
Spectrum of activity Yeast Candida spp. (except C. krusei) Cryptococcus spp. Dimorphic Blastomyces dermatitidis, Coccidioides spp., Histoplasma spp. Dermatophytes Microsporum spp, Epidermophyton spp and Trichophyton spp. Moulds: No activity (Aspergillus spp, Fusarium spp, Scedosporium spp ) Mucorales: No activity
Spectrumof activity Fungi Candida albicans Candida glabrata Candida krusei Cryptococcus spp Aspergillus fumigatus Aspergillus terreus Blastomyces spp Coccidioides spp Histoplasma spp Sporothrix spp Fusarium spp Scedosporium spp Mucorales Fluconazole +++ +/- - +++ - Itraconazole +++ + +/- ++ +++ Voriconazole +++ + +++ ++ +++ Posaconazole +++ + +++ ++ +++ - + +++ +++ ++ +++ +++ - - - +++ + + + - +/- +/- - +++ ++ ++ ++ + + + ++ ++ + - - - -
Drug Resistance Primary/intrinsic resistance C. krusei, C. glabrata-high MIC Acquired resistance 7% Candida species (U.S) 9-14% fluconazole-resistant C. glabrata Lack of source control Suboptimal treatment dose, duration Immunosuppression
Mechanism of azole resistance Four major mechanisms 1.Decreased drug concentration. The development of active efflux pumps results in decreased drug concentrations at the site of action. 2.Target site alteration. Prevents binding of azoles to the enzymatic site. 3.Up-regulation of target enzyme. Target enzyme up-regulation can be achieved through gene amplification, increased transcription rate, or decreased degradation of the gene product. 4.Development of bypass pathways. Replacement of ergosterol with the latter product leads to functional membranes and negates the action of azoles on the ergosterol biosynthetic pathway. More than 1 mechanism can be functioning in any given fungal strain with additive effects
Molecular mechanism of azole resistance Mutations in genes that encodes 14 -demethylase. Cyp51a ERG11 Upregulation of efflux pumps Cyp51b
Pharmacokinetics Bioavailability: >90% (oral), no acid effect. Linear pharmacokinetics Distribution: Effectively penetrates brain parenchyma/CSF, eye, skin tissue. Achieves concentrations in saliva, urine, vaginal secretions Protein binding: 11-12% Metabolism: Liver 10-11% ; zero order kinetics Half-life: 20-50 hours ; ~30 hours Excretion: Urine (61-88%); sweat
Pharmacokinetic profile Triazole Solubility Absorption Food effect - Bioavailability T1/2 (h) 20-50 Excretion Fluconazole High High >90% Renal Itraconazole Low Erratic ++ 55% 24-42 Hepatic Voriconazole High High ++ 96% 6 Hepatic Posaconazole High High ++ - - Hepatic
Target therapeutic range Drug Therapeutic range (mcg/mL) >25 Toxic level (mcg/mL) CYPs inhibited Rationale for TDM 5-flucytosine >100 n/a Clearance in renal disease Select patients only Variable absorption Fluconazole 4-20 not established not established 2C19, 3A4 Itraconazole >0.5 (localized) >1.0 (systemic) 1.0 -5.5 3A4 Voriconazole >6.0 2C9, 3A4 Nonlinear kinetics
Formulations Oral Capsules/Tablets: 50mg, 100mg, 150mg, 200mg Oral suspension : 50mg/5mL [35mL bottle] Parenteral 2mg/mL; 100-mL bottle
Clinical Indications A. Targeted therapy 1. Vaginal candidiasis 2. Candidal balanitis 3. Mucosal candidiasis 4. Tinea pedis, corporis, cruris, pityriasis versicolor and dermal candidiasis 5. Invasive candidal infection (candidaemia & disseminated candidiasis) 6. Cryptococcal infection(including meningitis) Pre-emptive treatment Cryptococcal antigenaemia Primary prophylaxis Immunocompromised patients (HSCT, pre-term babies etc.) Secondary prophylaxis Cryptococcal meningitis B. C. D.
Dose A. Targeted therapy 1. Vaginal candidiasis 2. Candidal balanitis 3. Mucosal candidiasis 4. Tinea pedis, corporis, cruris, pityriasis versicolor and dermal candidiasis 5. Invasive candidal infection (candidaemia & disseminated candidiasis) 6. Cryptococcal infection(including meningitis) Uncomplicated Adult & Child >16 years: 150mg single dose, by mouth Recurrent vulvovaginal candidiasis Adult & Child >16 years: 150mg 3 doses, 72hourly, then 150mg , every week , 6 months
Dose A. Targeted therapy 1. Vaginal candidiasis 2. Candidal balanitis 3. Mucosal candidiasis 4. Tinea pedis, corporis, cruris, pityriasis versicolor and dermal candidiasis 5. Invasive candidal infection (candidaemia & disseminated candidiasis) 6. Cryptococcal infection(including meningitis) Adult: 50-100mg, PO, daily, 7-14 days 14-30 days if associated with dentures Child: 3-6mg/kg, day 1, PO or IV Then 3mg/kg daily, 7-14 days
Dose A. Targeted therapy 1. Vaginal candidiasis 2. Candidal balanitis 3. Mucosal candidiasis 4. Tinea pedis, corporis, cruris, pityriasis versicolor and dermal candidiasis 5. Invasive candidal infection (candidaemia & disseminated candidiasis) 6. Cryptococcal infection(including meningitis) Adult & Child: 50mg , PO, Daily; 2-4 week Up to 6 weeks, tinea pedis Maximum duration, 6 weeks
Dose A. Targeted therapy 1. Vaginal candidiasis 2. Candidal balanitis 3. Mucosal candidiasis 4. Tinea pedis, corporis, cruris, pityriasis versicolor and dermal candidiasis 5. Invasive candidal infection (candidaemia & disseminated candidiasis) Adult: Child: 800mg day 1, then 400mg daily , 2-8 weeks 6-12mg/kg, Daily, PO or IV (Max 800mg daily)
Dose A. Targeted therapy Cryptococcal infection (including meningitis) Induction: Consolidation: Maintenance: 1200mg daily, IV or PO, 2 weeks 400-800mg daily, PO, 2-8 weeks 200mg , OD, PO, long-term Until immune reconstitution
Dose Pre-emptive treatment Cryptococcal antigenaemia Induction: Consolidation: Maintenance: 800mg daily,PO, 2 weeks 400mg daily, PO, 8 weeks 200mg daily, PO, long-term Until immune reconstitution
Dose A. Primary prophylaxis Immunocompromised patients Adult: 50mg-400mg daily, PO or IV 400mg for high risk patients (e.g. HSCT) Start treatment before anticipated onset of neutropenia Continue for 7 days after normal neutrophil count has been attained Child: 3-12mg/kg , daily, PO or IV: Max 400mg
Dose Secondary prophylaxis Cryptococcal meningitis Maintenance : 200mg PO daily ; long-term Until immune reconstitution
Renal impairment Decrease dosage by 50% for CrCl <50 cc/min Fluconazole is removed by hemofiltration. It should be administered after hemodialysis
Hepatic impairment Fluconazole is not extensively metabolised in the liver Dose adjustments not necessary for patients with hepatic failure Toxicity with related drugs
Pregnancy & Breastfeeding Pregnancy Multiple congenital abnormalities Avoid in pregnancy Breast-feeding Present in breastmilk Compatible with breastfeeding
Common Adverse Reactions Nausea Abdominal discomfort Diarrhoea Headache Elevated liver enzymes
Less frequent Side Effects GI Skin Alopecia Skin rash Toxic epidermal necrolysis Stevens Johnson syndrome Blood Leukopenia Thrombocytopenia Systemic/metabolic Hyperlipidaemia Hypokalaemia Hypersensitivity reactions Anaphylaxis Dyspepsia Vomiting Taste disturbance CNS Dizziness Seizures Liver Hepatitis Pruritus Cardiac QT prolongation Torsades de pointes
Summary Inhibits the conversion of lanosterol to ergosterol Active against Candida spp, Cryptococcus spp, dimorphic fungi, dermatophytes Resistance: Increased expression of efflux pumps, point mutations in ERG11 Excellent oral bioavailability. Administered PO or IV Hepatic adverse reactions
 undefined
undefined