Understanding Enzyme Inhibition in Biochemistry
Enzyme inhibition plays a crucial role in pharmacology and biochemistry by regulating enzymatic reactions. Inhibitors can be reversible or irreversible, affecting enzyme activity differently. Competitive, uncompetitive, and noncompetitive inhibition types are explained along with examples like diisopropylfluorophosphate as an irreversible inhibitor of chymotrypsin. The concept of inhibition constant (Ki) is highlighted, illustrating how inhibitors bind to enzymes. Understanding these mechanisms is vital for drug design and understanding biological processes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
BIOCHEMISTY Course No.-DTC-111, Credit Hours 2 (1+1) ENZYME INHIBITION BINITA RANI ASSOCIATE PROFESSOR (DAIRY CHEMISTRY) FACULTY OF DAIRY TECHNOLOGY S.G.I.D.T., BVC CAMPUS, P.O.- BVC, DIST.-PATNA-800014
Inhibitors are molecules that => resemble the substrate(s) or product(s) and bind to => active site => thus => they interfere with catalysis => slowing or halting enzymatic reactions. Many drugs are => reversible enzyme inhibitors. They have their physiological effect by => decreasing => the activity of a specific enzyme. For example, aspirin (acetylsalicylate) => inhibits the enzyme that catalyzes the first step in the synthesis of prostaglandins => compounds involved in many processes => including some that produce pain.
Concentration of inhibitor needed => to inhibit enzyme => depends on how tightly inhibitor binds to the enzyme. Inhibition constant (Ki) is used to describe => how tightly an inhibitor binds to an enzyme. Types of Inhibitors There are two broad classes of enzyme inhibitors: Irreversible Reversible
Irreversible irreversible inhibitors are those : that bind covalently with enzyme or destroy a functional group on an enzyme => that is essential for enzyme s activity, or that form => particularly stable noncovalent association. Formation of a covalent link between => an irreversible inhibitor and an enzyme is => common. For example => reaction of chymotrypsin with diisopropylfluorophosphate (DIFP) => irreversibly inhibits enzyme by binding with Ser195 in the active-site of chymotrypsin.
Diisopropylfluorophosphate as irreversible inhibitors of chymotrypsin
Reversible This type of inhibition involves => equilibrium between enzyme and inhibitor => equilibrium constant (ki) => being the measure of affinity of the inhibitor for the enzyme. This inhibition is further classified into three categories: Competitive Uncompetitive Noncompetitive.
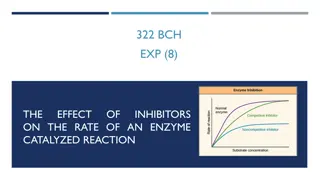
Competitive Inhibition Competitive inhibitors bind only to => free enzyme and to the same site as the substrate. Competitive inhibitors are => molecules that usually look like the substrate but can t undergo the reaction. At an infinite concentration of the substrate => competitive inhibitor cannot bind to the enzyme since => substrate concentration is high enough that => there is virtually no free enzyme present.
Since competitive inhibitors have => no effect on the velocity at saturating (Vmax) concentrations of the substrate => intercepts of the double reciprocal plots (1/Vmax) at all the different inhibitor concentrations are => the same. The lines at different inhibitor concentrations => must all intersect on the y axis at the same 1/Vmax.
At low concentrations of substrate ([S] << Km) => enzyme is predominantly in the E form. competitive inhibitor can combine with E => so the presence of the inhibitor => decreases => the velocity when => substrate concentration is low.
Competitive Inhibition Under competitive inhibition Vmax remains unchanged ; Km increases
Under competitive inhibition Vmax remains unchanged ; Km increases Example : Malonate is a competitive inhibitor of => succinate dehydrogenase . The enzyme uses succinate as its substrate but inhibited by malonate => which is structurally similar to succinate and => differs in having => one rather than two methylene groups.
Uncompetitive Inhibition If inhibitor combines only => with ES (and not E) => inhibitor exerts its effect only at => high concentrations of substrate at which => there is lots of ES around. This means that the increasing substrate concentration (S) => doesn t prevent => binding of the inhibitor.
Interestingly Km value is consistently smaller than Km value of the uninhibited reaction => which implies that => S is more effectively bound to the enzyme in the presence of the inhibitor. The sequence of this type of reaction is
This type of inhibition is often observed for enzymes => that catalyze the reaction between two substrates. Often an inhibitor that is => competitive against one of the substrates is found to give => uncompetitive inhibition => when the other substrate is varied. The inhibitor does combine at active site but => does not prevent => binding of one of the substrates (and vice versa).
In this type of inhibition => Vmax as well as Km both are decreased Uncompetitive Inhibition
Non-competitive Inhibition Compounds that reversibly bind with either the enzyme or the enzyme substrate complex are designed as => noncompetitive inhibitors and the following reaction describe these events. Non competitive Inhibition
Noncompetitive inhibition therefore differs from competitive inhibition in that => inhibitor can combine with ES, and S can combine with EI to form => in both instances EIS. This type of inhibition is not completely reversed by => high substrate concentration => since closed sequence will occur => regardless of the substrate concentration. Since inhibitor binding site is not identical to nor does it modify the active site directly => Km is not altered but Vmax is decreased.
For example => amino acid alanine noncompetitively inhibits => enzyme pyruvate kinase. Alanine is one product of => a series of enzyme- catalyzed reactions => first step of which is catalyzed by pyruvate kinase.

 undefined
undefined