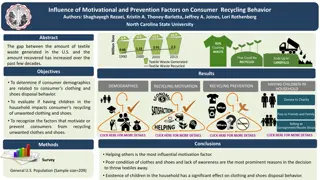
Understanding Plastics: Definition, History, and Recycling
Plastics are versatile materials that can be shaped when soft and hardened, replacing traditional materials like glass and wood. The history of plastics dates back centuries, with key milestones in the development of different types of plastics. Today, the Society of the Plastics Industry plays a crucial role in promoting recycling through the Resin Identification Code (RIC), categorizing plastics for efficient recycling. Explore the world of plastics, including the popular PET (polyethylene terephthalate) and its various applications in packaging and recycled products.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
What is a Plastic? Definition of Plastic from www.dictionary.com - any of a group of synthetic or natural organic materials that may be shaped when soft and then hardened, including many types of resins, resinoids, polymers, cellulose derivatives, casein materials, and proteins: used in place of other materials, as glass, wood, and metals, in construction and decoration, for making many articles, as coatings, and, drawn into filaments, for weaving.
History of Plastics References of plastic as far back as the Old Testament 1846 in Europe, Charles Sch nbein accidentally discovered nitrocellulose 1851 Ebonite (hard rubber) discovered, was the first thermosetting material to be prepared 1870 John W. Hyatt reacts nitrocellulose with camphor to make celluloid 1890s 1907 Formaldehyde Resins very important alternative to celluloid particularly casein. 1909 Leo Baekeland synthesized bakelite. Led to a class of plastics known as phenolic resins. 1930 s Wallace Carruthers, a Dupont Chemist, invents plastic polymer known as nylon. 1930-1940initial commercial development of today s major thermoplastics, PVC, LDPE, PS. The advent of WW II in 1939 brought plastics into great demand. 1945-1955 First Decade after WW II saw the development of PP, HDPE, and the growth of new plastic in many applications 1978 Linear low density polyethylene introduced (Density of 0.90 0.96) reduced the cost of their production drastically
Plastics Today Society of the Plastics Industry (SPI) founded in 1937. Developed a Resin Identification Code (RIC) in 1988 to help with the recycling of the different plastics available. There are 7 categories within the RIC. 1 PET (polyethylene terephthalate) 2 HDPE (high density polyethylene) 3 V or PVC (Vinyl or Poly (vinyl chloride)) 4 LDPE (low density polyethylene) 5 PP (polypropylene) 6 PS (polystyrene) 7 Other (mixed plastics)
1 PET (polyethylene terephthalate) (-CO-C6H4-CO-O-CH2-CH2-O-)n Packaging applications: Soft drink bottles, water bottles, beer bottles, mouthwash bottles, peanut butter containers, salad dressing containers, juice bottles, vegetable oil bottles Recycled products: Fiber, tote bags, new PETE containers for both food and non-food products, fabric for clothing, athletic shoes, luggage, upholstery, furniture, carpet, fiberfill for sleeping bags and winter coats, industrial strapping, sheet, and film, and automotive parts, such as luggage racks, headliners, fuse boxes, bumpers, grilles and door panels Note: the C6H4 highlighted in the molecular formula above represents a benzene ring. Benzene is generally considered a carcinogenic substance.
2 HDPE (high density polyethylene) (-CH2-CH2-)n Packaging applications: Milk containers, juice bottles, water bottles, bleach, detergent, and shampoo bottles, trash bags, grocery and retail carrying bags, motor oil bottles, butter and margarine tubs, household cleaner bottles, yogurt containers, and cereal box liners Recycled products: Drainage pipe, liquid laundry detergent bottles, oil bottles, pens, benches, doghouses, recycling containers, floor tile, picnic tables, fencing, lumber, and mailbox posts
3 - Vinyl or PVC (polyvinyl chloride) (-CH2-CHCl-)n Packaging applications: Window cleaner bottles, cooking oil bottles, detergent bottles, shampoo bottles, clear food packaging, wire and cable jacketing, medical tubing, with additional significant usage in household products and building materials, particularly siding, piping, and windows Recycled products: Binders, decking, paneling, mud flaps, roadway gutters, flooring, cables, speed bumps, and mats Note: The Cl (chlorine atom) in the molecular formula renders PVC a potentially toxic material when it is burned. The burning of PVC can result in the creation of dioxins, a material that is considered highly carcinogenic.
4 LDPE (low density polyethylene) ( - CH2 - CH2 - )n Packaging applications: Squeezable bottles, bread bags, frozen food bags, tote bags, clothing, furniture, dry cleaning bags, and carpet Recycled products: Film and sheet, floor tile, garbage can liners, shipping envelopes, furniture, compost bins, paneling, trash cans, lumber, landscaping ties Note: The molecular formulas for LDPE and HDPE are the same. The difference in the plastics is the density of the molecular chains. The density varies in the manner in which the polymeric chains form. In HDPE the chain is essentially one long continuous chain, allowing the strands to fold back upon one another and densely occupy space. In LDPE the chains have multiple branches, which interfere with a neatly organized packing of chains. Instead the packing is more disorganized, occupying more space and thus resulting in a lower density.)
5 PP( polypropylene) (-CHCH3-CH2-)n Packaging applications: Yogurt containers, syrup bottles, ketchup bottles, caps, straws, medicine bottles Recycled products: Signal lights, battery cables, brooms, brushes, auto battery cases, ice scrapers, landscape borders, bicycle racks, rakes, bins, pallets, and trays
6 PS (polystyrene) (-CHC6H5-CH2-)n Packaging applications: Plates, cups, cutlery, meat trays, egg cartons, carry-out containers, aspirin bottles, compact disc jackets Recycled products: Thermal insulation, light switch plates, egg cartons, vents, rulers, foam packing, carry-out containers Note: C6H5 in the molecular formula comprises a benzene ring. Benzene is generally considered a carcinogenic substance.
7 Other (misc. plastics) Properties: varies according to constituent resins Statistic: In 1999 there was minimal usage of resins in the 'other' category in plastic bottles. Description: The category of "Other" includes any resin not specifically numbered 1, 2, 3, 4, 5, or 6, or combinations of one or more of these resins. Packaging applications: Three and five gallon water bottles, certain food product bottles Recycled products: Plastic lumber, custom-made products
Videos 1944 Plastic Science and Chemistry video: http://www.youtube.com/watch?v=MKKFe4Y- 9EA
References SPI - http://www.plasticsindustry.org/AboutPlastics/content.cfm?ItemNumbe r=670 SPI - http://www.plasticsindustry.org/AboutPlastics/content.cfm?ItemNumbe r=823&navItemNumber=2144 How stuff works - http://science.howstuffworks.com/plastic1.htm EPA - http://www.epa.gov/osw/conserve/materials/plastics.htm Earth Odyssey - http://www.earthodyssey.com/symbols.html