Understanding pH and its Importance in Chemistry
Explore the concept of pH and its significance in chemistry, distinguishing between acids, bases, salts, and covalent compounds. Learn how to calculate pH, interpret acidic, basic, and neutral solutions based on the pH scale, and practice pH-related questions for a better understanding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
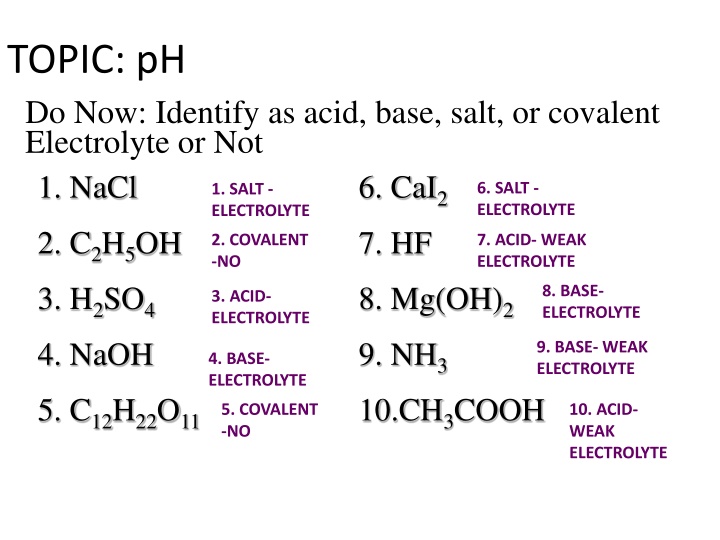
TOPIC: pH Do Now: Identify as acid, base, salt, or covalent Electrolyte or Not 1. NaCl 6. CaI2 7. HF 6. SALT - ELECTROLYTE 1. SALT - ELECTROLYTE 2. C2H5OH 3. H2SO4 4. NaOH 2. COVALENT -NO 7. ACID- WEAK ELECTROLYTE 8. Mg(OH)2 9. NH3 10.CH3COOH 8. BASE- ELECTROLYTE 3. ACID- ELECTROLYTE 9. BASE- WEAK ELECTROLYTE 4. BASE- ELECTROLYTE 5. C12H22O11 5. COVALENT -NO 10. ACID- WEAK ELECTROLYTE
pH scale P= power of H = Hydorgen Ranges from 0-14 measures H+ concentration [H+] the more H+, the more acidic the solution
Acid, Base, or Neutral Neutral solution: pH = 7 Acidic solution: pH LESS THEN 7 Basic solution: pH GREATER THEN 7
Every Acidic/Basic solution contains H+ and OH- H+1 > OH-1 OH-1 > H+1 For acids For bases when [H+] = [OH-] the substance is neutral so we can actually measure pH and pOH pH + pOH =14
Logarithmic scale (based on powers of 10) each decrease of one unit of pH represents a 10x increase in H+ concentration Ex: pH 4 is ten times more acidic then pH 5 Ex: pH 10 is ten times more basic then pH 9 Try to remember: The lower the pH, the higher the concentration of H+ ions
Lets Practice 1. When the pH of an aqueous solution is changed from 1 to 2, the Hydrogen ion concentration a) Decreases by a factor of 2 b) Decreases by a factor of 10 c) Increases by a factor of 2 d) Increases by a factor of 10 2. A solution with a pH of 2.0 has a hydronium ion concentration ten times greater than a solution with a pH of a) 1.0 b) 0.20 c) 3.0 d) 20 3. Which change in pH represents a hundredfold increase in the concentration of hydronium ions in a solution a) pH 1 to pH 2 b) pH 1 to pH 3 c) pH 2 to pH 1 d) pH 3 to pH 1
Calculating pH pH=-log [H+] [H+] = concentration Ex. 0.01M HCl has a pH of? This means you have .01 moles of H+ and .01 moles of Cl- per every 1 L pH = -log(0.01) pH=2 We are going to learn an easier way!
Because its based on powers of 10 there is a trick 10-x where x = pH If molarity of acid is .001M =10-3 pH = 3 If molarity of acid is .00001M = 10-5 pH = 5
pH of a base Now you are looking at OH- ions instead of H+ ONLY CAN CALC pOH pOH=-log [OH-] or our trick 10-x where x = pOH pH + pOH = 14 So 14 pOH = pH Ex. 0.01M NaOH has a pH of? This means you have .01 moles of OH- and .01 moles of Na+ per every 1 L pOH=2 pH = 14-2 = 12
Because its based on powers of 10 there is a trick If molarity of base is .001M = 10-3 pOH = 3 pH 14-3 = 11 If molarity of base is .00001M = 10-5 pOH = 5 pH 14-5 = 9
If pH = 4 1 x 10-4 M [H+1] = ? pH + pOH = 14 4 + X = 14 X =10 pOH = ? [OH-1] = ? 1x10-10 M = [OH-1]
If the [OH-1] = 1 X 10-3 M pOH = 3 pOH = ? pH = ? pH + pOH = 14 X + 3 = 14 X = 11 [H+1] = ? 1x10-11 M
If the [H+1] = 1 x 10-5 M The pH = ? =5 The pOH = ? pH + pOH =14 5+x =14 X=9 1x10-9M The [OH-] = ?
Calc pH for .01M HCl 10-2 = 2 so pH = 2 .01M NaOH 10-2 = 2 so pOH = 2 14-2 12 pH=12
