Understanding Enterotomy in Small Intestine Surgery
Enterotomy is a surgical procedure performed in the small intestine for various indications such as foreign bodies and biopsy samples. Learn about the anatomy, indications, and closure techniques involved in enterotomy surgery.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
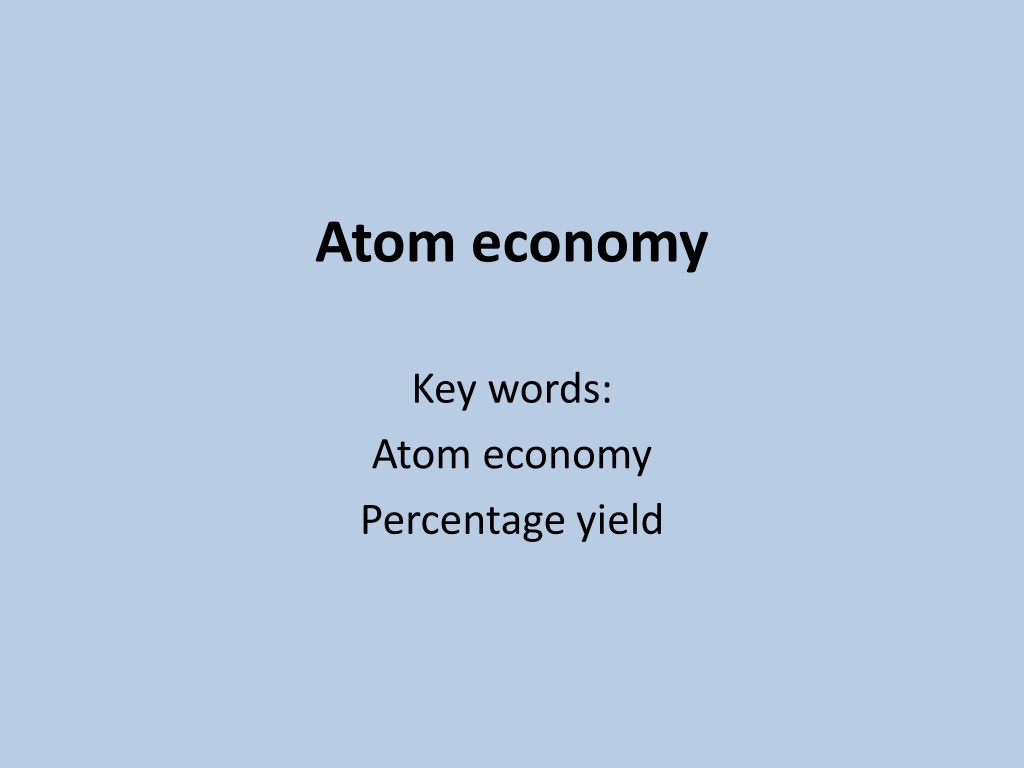
Atom economy Key words: Atom economy Percentage yield
Learning objectives Define the atom economy of a reaction and describe the benefits of developing chemical processes with a high atom economy. Explain that addition reactions have an atom economy of 100%, whereas substitution reactions are less efficient. Carry out calculations to determine the atom economy of a reaction. Explain that a reaction may have a high percentage yield but a low atom economy.
Percentage yield is used to assess the efficiency of a chemical reaction. Calculating the percentage yield only tells part of the story. A reaction may not only produce the desired product, but by products as well What happens to the by-products? Considered as a waste and disposed of Costly Potential environmental problems May be sold on or used elsewhere in the plant Preserves Earth s resources Minimises waste
Equation ????????? ???? ?? ??????? ??????? ??? ?? ????????? ?????? ?? ??? ????????? 100 ???? ??????? =
Atom economy considers not only the desired product, but also all the by-products of a chemical reaction. It describes the efficiency of a reaction in terms of ALL the atoms involved . A reaction with a high atom economy uses atoms with minimal waste We are now much more aware of our environment. By using processes with a higher atom economy, chemical companies can reduce the amount of waste produced. This is good news, especially as we are running out of landfill sites. It has also been suggested that about 5-10% of the total expenditure of a chemical company goes on waste treatment
1. Calculate the molecular mass of the desired product 2. Divide this by the sum of the molecular masses of all products
The type of reaction used for a chemical process is a major factor in achieving a higher atom economy. Addition reactions have an atom economy of 100% Reactions involving substitution or elimination have an atom economy less than 100% This doesn t mean that we should never carry out substitution or elimination reactions but we need to find a use for all the products to improve their atom economy. This can be problematic when the undesired products are toxic