Understanding Citral: A Monoterpene Aldehyde Compound
Citral is a natural acyclic compound found in lemon grass and citrus fruits, widely used in perfume, flavor, and medicinal industries. Learn about its isolation, synthetic procedures, and chemical structure through this informative guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
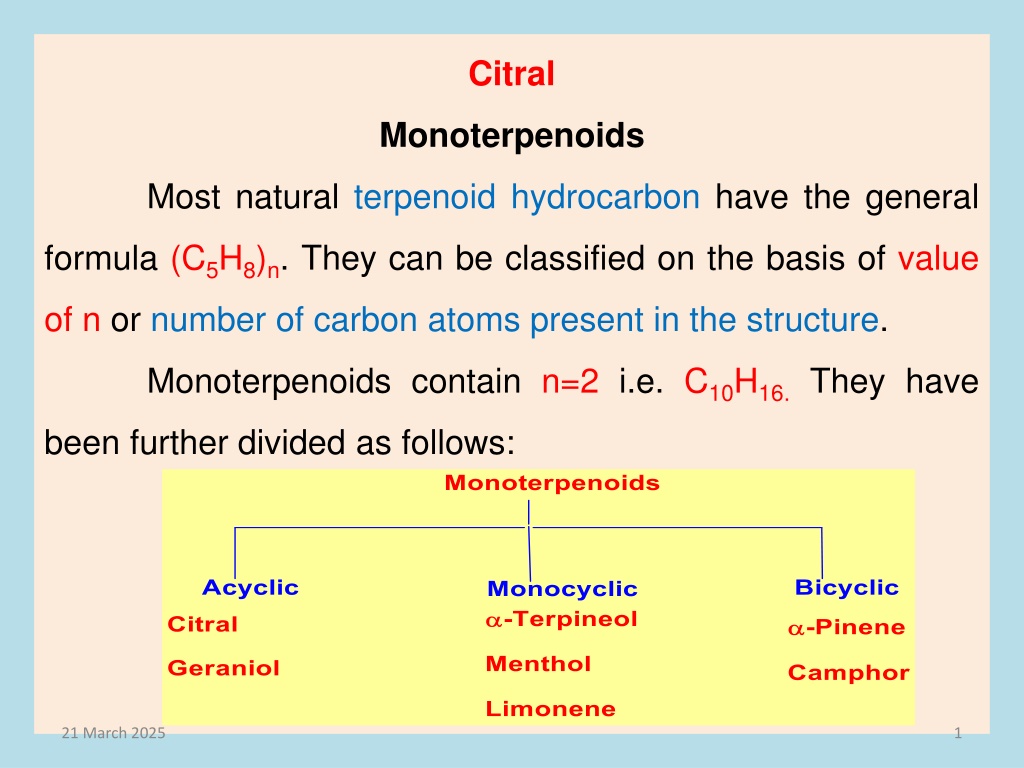
Citral Monoterpenoids Most natural terpenoid hydrocarbon have the general formula (C5H8)n. They can be classified on the basis of value of n or number of carbon atoms present in the structure. Monoterpenoids contain n=2 i.e. C10H16.They have been further divided as follows: 21 March 2025 1
Acyclic Monoterpenoids Citral monoterpenoids an aldehyde of the terpene series and is an isomeric mixture of geranial and neral which is obtained from lemon grass oil. It is also present in the oil of citrus fruits (Citron, Orange, and Lemon ). It is pale yellow liquid b.p. 228 0C having lemon like smell. Citral is naturally occurring aliphatic acyclic 21 March 2025 2
It is used extensively in the perfume and flavor industry to stimulate lemon like odour and for manufacture of vitamin A. Recently citral has become important as a drug for reducing blood pressure. It is optically active. Isolation Citral is obtained by fractional distillation of lemon grass oil under reduced pressure. It is treated with sodium bisulphate which on hydrolysis with sodium carbonate gives pure citral. 21 March 2025 3
Principal constituent of lemongrass oil can be isolated by fractional distillation. Citral is isolated by distillation from lemongrass oil and from Litsea cubeba oil . Citral is usually isolated from the citral-containing oil by chemical means or by chemical synthesis (from beta-pinene) 21 March 2025 4
Currently, the most important synthetic procedures are vapor-phase dehydrogenation and oxidation of geraniol or geraniol-nerol mixtures. Catalytic dehydrogenation under reduced pressure using copper catalysts is preferred. Dehydrolinalool is produced on a large scale from 2-methyl-2-hepten-6-one and acetylene and can be isomerized to citral in high yield by a number of catalysts. 21 March 2025 5
Structure 1. Its molecular formula is C10H16O. 2. Presence of double bond It adds two molecules of bromine or hydrogen to give citral tetrabromide or tetrahydrocitral respectively which indicates that it contains two double bonds. 21 March 2025 6
Citral on ozonolysis followed by decomposition with Zn / H2O yields acetone, laevulinic aldehyde and glyoxal which indicates that it is an acyclic compound having two double bonds. 21 March 2025 7
3. Presence of aldehydic group Following reactions indicate that it contains aldehydic group: i. On treatment with hydroxylamine it forms oxime. ii. It adds one molecule of sodiumbisulphite. 21 March 2025 8
iii. It reacts with phenylhydrazine to give phenylhydrazone. iv. Citral on reduction with sodium amalgam gives an alcohol Geraniol C10H18O. 21 March 2025 9
v. Citral on oxidation with silver oxide gives geranic acid containing same number of carbon atoms. vi. It reduces Fehling s solution and Tollen s reagent. 21 March 2025 10
4. Semmler observed that on treatment with potassium hydrogen sulphate forms p-cymene having cyclic aromatic character. The formation of p-cymene and ozonolysis products of citral indicates that the carbon skeleton of this compound contains two isoprene units which are joined head to tail. This reaction also indicates the position of methyl and isopropyl groups in citral. 21 March 2025 11
5. Tiemann and Semmler observed that citral on oxidation with alkaline potassium permanganate followed by chromic acid gives acetone, oxalic acid and laevulic acid. They proposed that citral possess following structure which explain the oxidation products. 21 March 2025 13
6. The above structure was supported by work of Verley who observed that citral on treatment with aqueous potassium carbonate yields 6-Methylhept-5-en-2-one and actaldehyde. The formation of these products is readily explained by assuming the above structure for citral which undergoes cleavage at the , - double bond. This reaction is characteristic of , -unsaturated oxo compounds. 21 March 2025 14
Thus on the basis of above observation citral possess following structure which explains all the reactions cited above. 21 March 2025 15
The above structure of citral was confirmed by its synthesis. 21 March 2025 17
Barbier and Tiemann synthesis 21 March 2025 18
Geometrical isomerism of Citral shows geometrical geometrical isomers are possible. The functional group (aldehyde) is trans or cis with respect to the methylene group of main chain. Both isomers occur in natural citral. Both forms of citral have been obtained. Citral a or trans form or E also known as Geranial b.p. 118-119 0C and citral b or cis form or Z also known as Neral b.p. 117-118 0C. It is found that corresponding alcohols geraniol and nerol undergo ring closure with dil H2SO4 to -Terpineol. The ring closure from Nerol to -Terpineol is nine times faster than closure of geraniol to -Terpineol. Citral isomerism and two 21 March 2025 19
