Understanding Antibodies: Structure, Production, and Labeling Techniques
Antibodies, or immunoglobulins, are essential components of the immune system. Learn about their structure, production methods, and different immunolabeling techniques. Discover how antibodies target specific biomolecules and their applications in immunofluorescence.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
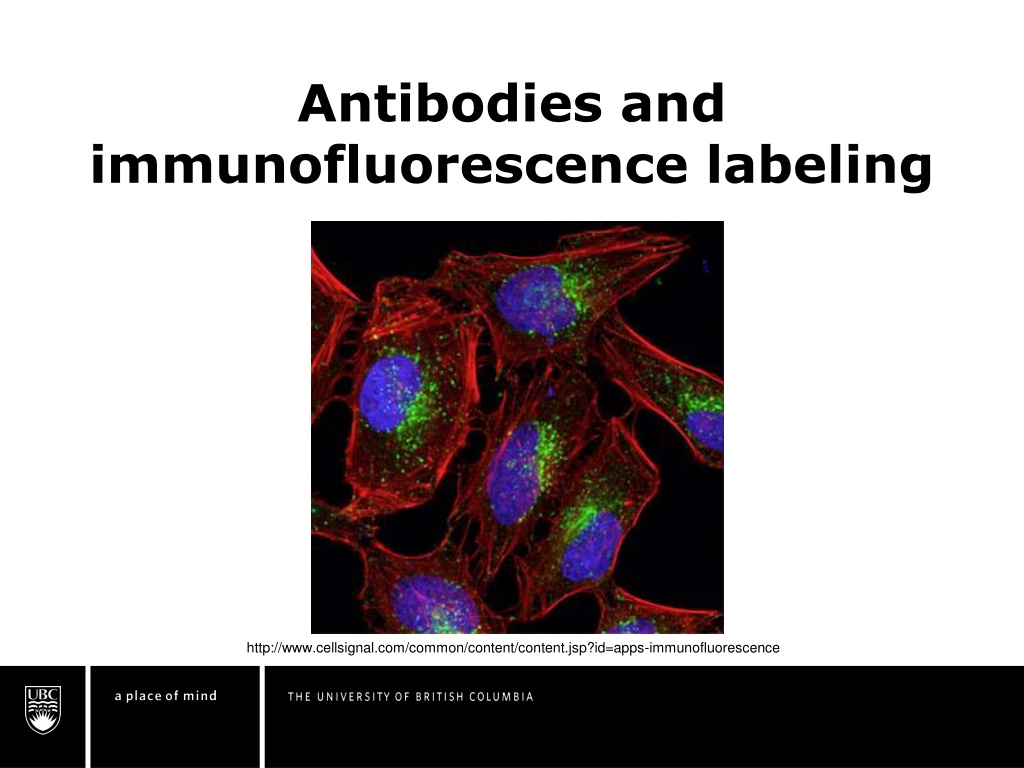
Antibodies and immunofluorescence labeling http://www.cellsignal.com/common/content/content.jsp?id=apps-immunofluorescence
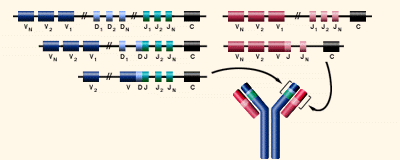
What is an antibody? Antibodies, or immunoglobulins, are glycoproteins composed of 2 heavy and 2 light chains. Both light and heavy chains have different variable regions depending how the DNA is arranged and can form more than 15,000,000 combinations Somatic Recombination is the mechanism by which some DNA is removed from the antibody coding region Variable regions http://www.biology.arizona.edu/immunology/tutorials/immunology/05t.html
How do you create a specific antibody? 1. Immunize mouse to desired antigen 2. Isolate B cells (mortal) from spleen 3. Cultivate myeloma cells (immortal) 4. Fuse both cell types 5. Separate individual cells and cultivate 6. Screen for antibody production 7. Multiply and harvest successful hybridoma cells http://en.wikipedia.org/wiki/Hybridoma_technology
Different immunolabeling techniques Immunofluorescence straining: antibodies are conjugated with a fluorescent dye that can be excited to emit detectable light Immunoenzymological staining: antibodies are conjugated with an enzyme that catalyzes the transitions of an added substrate into an insoluble particle that can be localized Immunocolloidal gold staining: antibody is conjugated with gold that is used as a marker
QUIZ 1! 1. Why can different antibodies bind to so many different biomolecules? 2. Why do you think the B cells are fused with myeloma cells? 3. What is the main difference between immunofluorescence staining, immunoenzymological staining, and immunocolloidal gold staining?
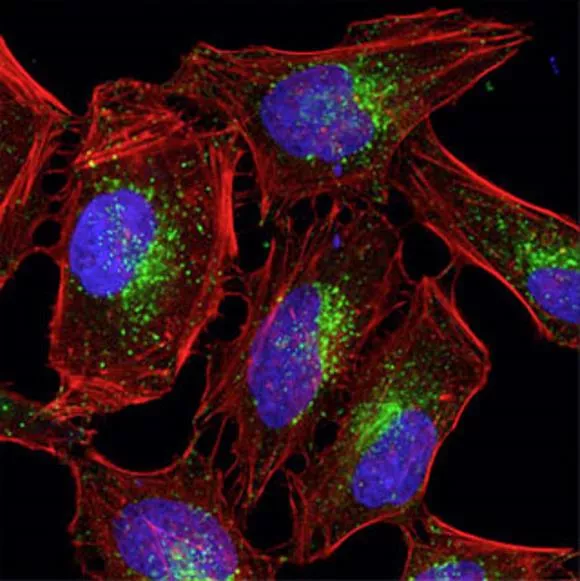
Function of immunofluorescence Antibodies target specific biomolecules (antigens) and reveal their distribution and density throughout the sample via conjugated fluorophore http://alizeepathology.com/samples/1 Green = AlexaFluor 488nm dye conjugated to actin; Blue = DAPI; Red = auto-fluorescence
Primary/Direct immunofluorescence Uses a single type of antibody The primary antibody binds directly to target biomolecule (antigen) and fluoresces via the attached fluorophore http://www.abcam.com/secondary-antibodies/direct-vs-indirect-immunofluorescence
Secondary/Indirect immunofluorescence Uses 2 antibody types The primary antibody binds directly to the target biomelocule (antigen) but has no attached fluorophore The secondary antibody binds to the primary antibody and fluoresces via the attached fluorophore http://www.abcam.com/secondary-antibodies/direct-vs-indirect-immunofluorescence
Example of primary/direct immunofluorescence SLBP (stem-loop binding protein) antibody binding to hairpin loops on mRNA in Hep G2 cells (liver tissue with hepatocellular carcinoma) http://www.scbt.com/datasheet-362617.html
Example of secondary/indirect immunofluorescence Smooth muscle antibody binding to actin, vimentin, desmin, and tubulin (all microfilaments) in Hep G2 cells (liver tissue with hepatocellular carcinoma) http://www.birmingham.ac.uk/facilities/clinical-immunology- services/autoimmunity/liver-associated-antibodies/Liver-antibodies.aspx
Primary versus Secondary immunofluorescence TIME Primary: Secondary: Less steps require less time Additional steps require additional time.
Primary versus Secondary immunofluorescence COMPLEXITY Primary: Secondary: Less steps in the protocol makes the experiment less complex Additional steps, selection of secondary antibodies, and potential background interactions add additional complexity
Primary versus Secondary immunofluorescence COST Primary: Secondary: Primary antibodies with an attached fluorophore (conjugated) are typically more expensive than unconjugated primary antibodies Secondary antibodies are less expensive and can used against different primary antibodies
Primary versus Secondary immunofluorescence SENSITIVITY Primary: Secondary: Less conjugated antibodies bind overall, so the signal is less intense Several secondary antibodies bind to the primary antibody and produce an amplified signal
Primary versus Secondary immunofluorescence FLEXIBILITY Primary: Secondary: Pre-conjugated primary antibodies have limited flexibility Ability to use different secondary antibodies increase flexibility of experiment
Primary versus Secondary immunofluorescence SUMMARY! Primary: Secondary: Less time required Less complexity Higher cost Less sensitivity Less flexibility Higher time required Higher complexity Less cost Higher sensitivity Higher flexibility
QUIZ 2! 1. Is primary or secondary immunofluorescence more complex? 2. If you were attempting to detect a biomolecule that likely has very low concentration in the sample, would you choose to use secondary or primary immunofluorescence?
Immunofluorescence protocol for staining cell cultures General Procedure 1. Coat coverslips in polyethyleneimine (for increased adhesion) 2. Rinse coverslips with water 3. Dry coverslips 4. Grow cells on glass coverslips 5. Rinse with Phosphate-based saline (PBS)
Immunofluorescence protocol for staining cell cultures Fixation 1. Either Incubate cells with 100% methanol or 4% (para)formaldehyde in PBS 2. Wash cells three times with cold PBS
Immunofluorescence protocol for staining cell cultures (Optional) Permeabilization 1. Incubate samples with PBS and 0.1-0.25% Triton X-100 (a detergent that improve antibody penetration) 2. Wash cells with PBS three times
Immunofluorescence protocol for staining cell cultures Blocking and incubation 1. Incubate cells with -1% BSA (bovine serum albumin) -22.52mg/ml glycine (reacts with any excess formadehyde) in PBS + tween 20 (a surfactant) for 30 minutes 2. Incubate cells with primary antibodies in 1% BSA in PBS + tween 20 for 1 hour at room temperature
Immunofluorescence protocol for staining cell cultures Blocking and incubation 3. Wash cells three times in PBS 4. Incubate cells with secondary antibody in 1% BSA for 1 hour at room temperature in the dark 5. Wash antibody solution three times with PBS in the dark
Immunofluorescence protocol for staining cell cultures Mounting 1. Mount coverslip with a drop of mounting medium 2. Seal coverslip 3. Store in darkness
LAST QUIZ! 1. If you were examining a human cell culture with indirect immunofluorescence using bovine primary antibodies and rabbit secondary antibodies, what type of albumin serum would you use when blocking and incubating the cell culture?
General references 1. http://www.bio.davidson.edu/genomics/method/IMF.html 2. http://www.abcam.com/secondary-antibodies/direct-vs- indirect-immunofluorescence 3. http://www.microscopemaster.com/immunofluorescence- microscopy.html 4. http://en.wikipedia.org/wiki/Antibody 5. http://atlasgeneticsoncology.org/Genes/CEACAM1ID40044 ch19q13.html 6. http://www.biology.arizona.edu/immunology/tutorials/immun ology/05t.html 7. http://rockland-inc.com/BasePage.aspx?id=41002 8. http://www.piercenet.com/method/overview-antibody- production-purification 9. http://www.scbt.com/datasheet-362617.html
Academic References 1. Agrawal A, Godar DE. Simultaneous Detection and Semiquantification of DNA Damage in Normal and Apoptotic Cells: Triple-Immunofluorescent Labeling Using DAPI, Antibodies, and TUNEL. Applied Immunohistochemistry & Molecular Morphology. 2012;20:402- 409. 2. Bordeaux J, Welsh AW, Agarwal S, et al. Antibody validation. BioTechniques. 2010;48:197-209. 3. Galluzzi L, Aaronson SA, Abrams J, et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009;16:1093-1107. 4. Zinchuk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: Pushing pixels to explore biological phenomena. Acta Histochem Cytochem. 2007;40:101-111.
Indirect fluorescence picture example Skin and vaginal tissues exposed to UV damage at time of damage (a,b), 1 hour post-damage (c,d), and 24 hours post damage (c,f) DAPI = blue (nuclei) TUNEL = green (apoptosis) CPD (cyclobutane pyrimidine dimers) antibody = red (DNA damage) Agrawal A, Godar DE. Simultaneous Detection and Semiquantification of DNA Damage in Normal and Apoptotic Cells: Triple-Immunofluorescent Labeling Using DAPI, Antibodies, and TUNEL. Applied Immunohistochemistry & Molecular Morphology. 2012;20:402-409