Tissue Culture and Explant Selection in Plant Micropropagation
Tissue culture, or micropropagation, involves propagating plants from small tissue pieces in sterile culture. The process of dedifferentiation and redifferentiation allows for the regeneration of whole plants from individual cells or explants. Explants, selected from meristematic tissue, develop callus that can lead to the formation of roots, shoots, or somatic embryos. Factors like explant age, plant quality, and ultimate goals impact the success of tissue culture.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
TISSUE CULTURE Tissue culture (often called micropropagation) is a special type of asexual propagation where a very small piece of tissue (shoot apex, leaf section, or even an individual cell) is excised (cut-out) and placed in sterile (aseptic) culture in a test tube, petri dish or tissue culture container containing a special culture medium. THE CAPACITY TO GENERATE A WHOLE PLANT FROM ANY CELL OR AN EXPLANT IS TERMED AS TOTIPOTENCY. The phenomenon of the reversion of mature cells to the meristematic state leading to the formation of callus is called dedifferentiation. The component cells of callus have the ability to form a whole plant, a phenomenon described as redifferentiation.
EXPLANT A SMALL PIECE OF THE DESIRABLE PLANT IS SELECTED. GENERALLY MERISTEMATIC TISSUE OR INTERNODAL SEGMENTS OF THE PLANT IS SELECTED FOR MICROPROPAGATION. THE SELECTED PLANT TISSUE IS CALLED AS EXPLANT.
Introduction The explant is a piece of plant tissue placed into tissue culture. An explant can develop a callus as a wound response that consists of unorganized, dividing cells. Additionally, callus can be produced without wounding by germinating some seeds on a medium containing a plant growth regulator like 2,4-D. Callus cells vary in size, shape, pigmentation, and sometimes in genetic expression.
Introduction These cells are highly differentiated in that they have a large central vacuole and the nucleusis to the side. This is in contrast to undifferentiated, meristematic cells that are isodiametric, small, lack a prominent vacuole, are cytoplasmic, and have a large central nucleus. These meristematic cells are sometimes initiated in callus masses and are referred to as meristemoid regions. Meristemoids can give rise to adventitious roots, shoots, or somatic embryos.
Introduction Factors in explant selection include consideration of the following: 1.Physiological or ontogenic age of the organ that is to serve as the explant source 2.Season in which the explant is obtained 3.Size and location of the explant 4.Quality of the source plant 5.Ultimate goal of cell culture
1. EXPLANT AGE The age of the explant can be very important, as physiologically younger tissue is generally much more responsive in vitro. In many cases, older tissue will not form callus that is capable of regeneration. In addition, younger tissue is usually the newest formed and is generally easier to surface disinfect and establish clean cultures.
2. EXPLANT SIZE The explant size has an effect on the response of the tissue. Generally, the smaller the explant, the harder it is to culture. The culture medium usually has to have additional components. The larger explants probably contain more nutrient reserves and plant growth regulators to sustain the culture.
2. EXPLANT SIZE Plants have different hormonal balances throughout the plant and depending on the location of the explant, the explant can have a different endogenous level of plant growth regulators. Internal differences in hormone balance in the tissue can result in varying in vitro responses.
3. PLANT QUALITY It is advisable to obtain explants from plants which are healthy as compared to plants under nutritional or water stress or plants which are exhibiting disease symptoms. In some instances such as when establishing virus-free plants, the plant from which the explant is harvested has a virus or multiple viruses.
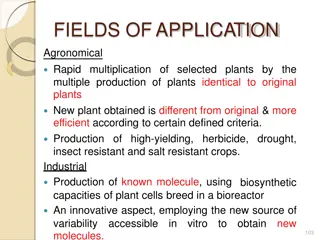
4. GOAL Depending on what type of a response is desired from the cell culture, the choice of explant tissue will vary. Any piece of plant tissue can be used as an explant (Fig. 4.1). For example, if clonal propagation is the goal, then the explant will usually be a lateral or terminal bud or shoot. For callus induction, pieces of the cotyledon, hypocotyl, stem, leaf, or embryo are usually used.
GOAL Excellent explants for callus induction are seedling tissues from aseptically germinated seeds or immature inflorescences. Leaf tissue from the aseptically germinated seed is a good source of tissue for protoplast isolation. To produce haploid plants or callus, the anther or pollen is cultured.
Plant Tissue Culture Media What s really important?
Plant Tissue Culture Why does it work? Plant cells Dedifferentiate Plant cell division- Somatic cells are diploid Mitosis Chromosomes duplicate and divide Meiosis The process of forming sex cells, 2n splits and become 1n gametes
Major Constituents Salt Mixtures Organic Substances Natural Complexes Inert Supportive Materials Growth Regulators
Macro-nutrient salts What the ? NH4NO3 KNO3 CaCl2 -2 H2O MgSO4 -7 H2O KH2PO4 FeNaEDTA H3BO3 MnSO4 - 4 H2O ZnSO4 - 7 H2O KI Na2MoO4 - 2 H2O CuSO4 - 5 H2O CoCl2 - H2O Ammonium nitrate Potassium nitrate Calcium chloride (Anhydrous) Magnesium sulfide (Epsom Salts) Potassium hypophosphate Fe/Na ethylene-diamine-tetra acetate Boric Acid Manganese sulfate Zinc sulfate Potassium iodide Sodium molybdate Cupric sulfate Cobaltous sulfide
Macronutrient salts Nitrogen Influences plant growth rate, essential in plant nucleic acids (DNA), proteins, chlorophyll, amino acids, and hormones. Phosphorus Abundant in meristematic and fast growing tissue, essential in photosynthesis, respiration. Potassium Necessary for cell division, meristematic tissue, helps in the pathways for carbohydrate, protein and chlorophyll synthesis.
Macronutrient salts Calcium - Involved in formation of cell walls and root and leaf development. Participates in translocation of sugars, amino acids, and ties up oxalic acid (toxin). Iron -Involved in respiration , chlorophyll synthesis and photosynthesis. FeNaEDTA = sodium salt of EDTA sequesters iron, making it available to plants. Magnesium - Involved in photosynthetic and respiration systems. Active in uptake of phosphate and translocation of phosphate and starches.
Micronutrient salts Sulfur - Involved in formation of nodules and chlorophyll synthesis, structural component of amino acids and enzymes. Manganese - Involved in regulation of enzymes and growth hormones. Assists in photosynthesis and respiration.
Micronutrient salts Molybdenum - Involved in enzymatic reduction of nitrates to ammonia. Assists in conversion of inorganic phosphate to organic form. Zinc - Involved in production of growth hormones and chlorophyll. Active in respiration and carbohydrate synthesis. Boron - Involved in production of growth hormones and chlorophyll. Active in respiration and carbohydrate synthesis. Copper -Involved in photosynthetic and respiration systems. Assists chlorophyll synthesis and used as reaction catalyst.
Organic Compounds Carbon Sources Sucrose, sometimes Glucose or Fructose (Plants Need Carbon) Vitamins Adenine part of RNA and DNA Inositol part of the B complex, in phosphate form is part of cell membranes, organelles and is not essential to growth but beneficial Thiamine essential as a coenzyme in the citric acid cycle.
Still other organics Organic Acids Citric acid (150 mg/l) typically used with ascorbic acid (100 mg/l) as an antioxidant. Can also use some of Kreb Cycle acids Phenolic compounds Phloroglucinol - Stimulates rooting of shoot sections
Natural Complexes Coconut endosperm Fish emulsion Protein hydrolysates Tomato juice Yeast extracts Malt extract Potato agar
Charcoal Activated charcoal is used as a detoxifying agent. Detoxifies wastes from plant tissues, impurities Impurities and absorption quality vary Concentration normally used is 0.3 % or lower Charcoal for tissue culture acid washed and neutralized never reuse
Growth regulators auxin - Roots cytokinin - Shoots gibberellin Cell Enlargement abscisic acid Plant stress hormone ethylene BAD!
Auxins Callus formation, rooting of cuttings, and the induction of adventive embryogenesis IAA IBA NAA 2,4-D 2,4,5-T Picloram
Cytokinins -Enhances adventitious shoot formation BA 2iP Kinetin Zeatin
Gibberellin Not generally used in tissue culture Tends to suppress root formation and adventitious embryo formation
Abscisic Acid Primarily a growth inhibitor but enables more normal development of embryos, both zygotic and adventitious
Ethylene Question is not how much to add but how to get rid of it in-vitro Natural substance produced by tissue cultures at fairly high levels especially when cells are under stress Enhances senescense Supresses embryogenesis and development in general
Hormone Combinations Callus development Adventitious embryogenesis Rooting of shoot cuttings Adventitious shoot and root formation
ASEPTIC TECHNIQUE For Explants Explants require surface-disinfestation before they can be placed in culture on the nutrient agar. This is generally accomplished by using diluted commercial chlorine bleach. Some explants, such as very small seeds or fern spores, are surface disinfected in conical, capped centrifuge tubes and require centrifugation to pellet the seeds and decanting off the solutions with a pipette.
There are 3 principal ways to kill off surface contaminants. oxidant action Active halogen Heavy metal poisoning There is always a trade-off between killing the surface contaminants and killing the explant. As far as possible, cut surfaces should be protected.
Sterilants used time 10-20% v/v 10-20 mins 10-20% v/v 10-20 mins 1% v/v 10 mins 0.1% w/v 10-30 mins 1% w/v 10-30 mins Conc Action oxidant / Halogen oxidant / Halogen oxidant Heavy metal Heavy metal NaOCl CaOCl H2O2 HgCl2 AgNO3 Antibiotics are rarely used since many are bacteriostatic and can cause mass overgrowth of cultures when they are removed. There are no antifungal compounds that are proven to be innocuous.
ASEPTIC TECHNIQUE For Explants Explants that float in the disinfectant can be wrapped in squares of cheesecloth to prevent their floating out of the beaker or test tube as the solutions are changed. A general procedure for preparing the explant is as follows:
ASEPTIC TECHNIQUE For Explants 1.Wash the explant in warm, soapy water and rinse in tap water. This procedure is very beneficial for stem, leaf, and shoot tip explants from the field or greenhouse because it removes surface contaminants. 2.Sometimes a brief alcohol rinse or swabbing with alcohol wetted cheesecloth is appropriate especially with surfaces that are hairy or coated with thick wax.
ASEPTIC TECHNIQUE For Explants 1.Immerse the explant in the chlorine bleach solution, which should always be made up fresh. Always add 1-2 drops of Tween-20, detergent, or other wetting agent per 100 ml of bleach solution. A 10% bleach solution is prepared by adding 10 mI of chlorine bleach to a graduated cylinder and diluting with water to 100 ml. When disinfesting in culture tubes, pour the bleach solution into the culture tubecap and then into the culture tube. This helps to disinfest the cap.
ASEPTIC TECHNIQUE For Explants 1.Place your hand over the tube and mix. Cap and agitate occasionally for 5-30 min for disinfestation. Chlorine bleach can age and lose its effectiveness. For this reason small containers of commercial bleach are preferable to large ones. 2.Decant the bleach solution and rinse the explant in sterile water three to five times. This step is carried out in a transfer hood (laminar flow cabinet).
ASEPTIC TECHNIQUE For Explants Some tissues are more difficult than others for establishing clean cultures. The concentration of chlorine bleach and the length of time the tissue is in the bleach can vary. Delicate, succulent tissue may be clean after 10 min in a 10% solution. Fern spores may only require a 3-5% bleach for 3-4 min.
ASEPTIC TECHNIQUE For Explants Some seeds may require 30-50% bleach for 20-60 min. Any cut explant such as a stem or leaf that is surface sterilized will almost always shows tissue damage from the surface sterilization. These damaged pieces should be removed before culture.
ASEPTIC TECHNIQUE For glassware, media and working area Autoclave all glass wares/ Oven dry and sterilize at 180 C for 2 hour. you can add antibiotics (after autoclave) to prevent bacterial contamination but no chemical to control fungal contamination as it a eukaryote just like your plant. Sterilize the flame hood properly .Put the UV on in the hood for 30 min before you work in the laminar hood, check if the filters of your hood are Ok and working, wipe the hood bench with alcohol and bleach and wear gloves and wipe them with alcohol. Anything inside the hood should be wiped with ethanol, still use a flame inside the hood, as flame is the best sterile condition, autoclave long enough, at least for 20-30 min at 121C. Keep the head out of the hood and try to maintain the hood room as clean as possible with minimum entries. And don't forget to change the filters of the hood, where the air flow goes in, after certain length of time. To make sure the air flow inside the hood is sterile, the hood need to be certified every period of time from a specialty company. They have the adequate equipment for detecting and measuring the air quality in the hood. Minimum rush at working place is the most helpful. Monitor the culture and remove the infected parts or plants to avoid spread.
ASEPTIC TECHNIQUE Contamination that results from improperly sterilized tissue will generally arise from the explant and be located the medium adjacent to the explant. Contamination due to poor technique generally will appear over the entire agar surface. Contamination of cultures by fungi appears fuzzy growth. Bacterial contamination appears as smooth pink, white, or yellow colonies.
ASEPTIC TECHNIQUE If contamination is due to poor technique, contaminated transfer hood filters, or improperly sterilized media it will be scattered on or in the medium. Contamination from insects will generally appear as tracks across the medium, which are visible due to bacterial or fungal growth on the insect tracks.
Factors affecting tissue culture Environmental factors (Controlled) Light intensity Photoperiod Temperature Sterility