Study on GLE/PIB Treatment in Renal Impairment Patients
This study explores the efficacy and safety of GLE/PIB treatment in patients with renal impairment and chronic kidney disease stages 3b, 4, or 5. The design involves non-randomised, open-label treatment for HCV genotype 1-6 patients aged 18 years and above. Primary endpoint includes SVR12, with baseline characteristics and adverse events also monitored. Results indicate high SVR rates and manageable adverse events in this patient population.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
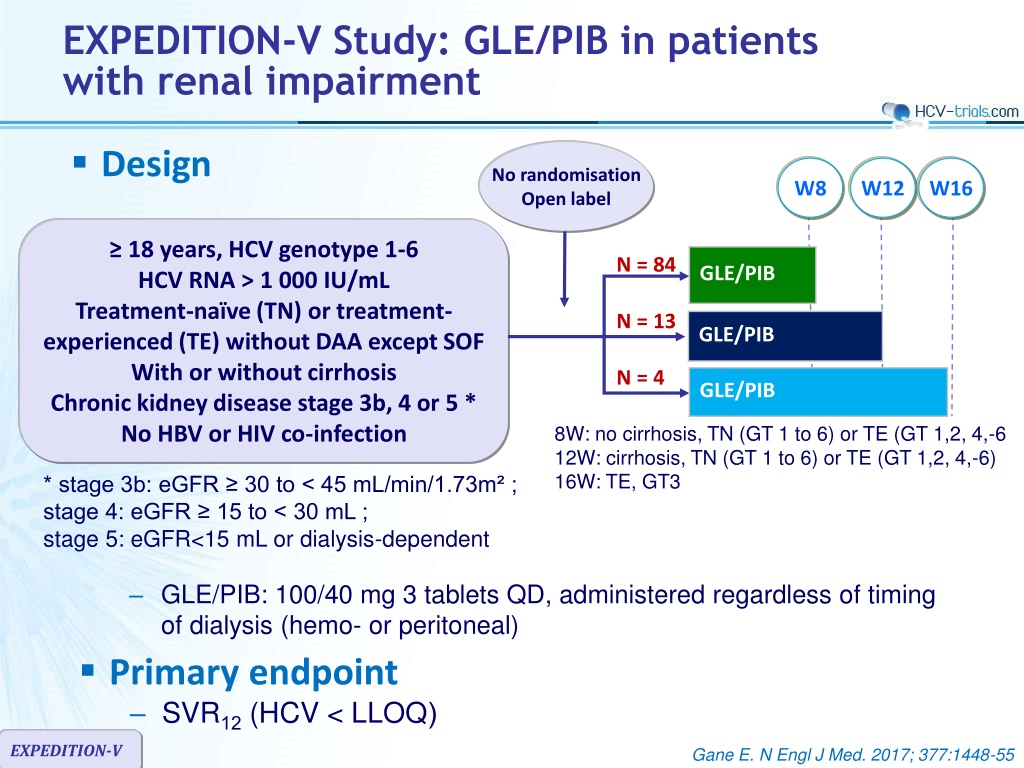
EXPEDITION-V Study: GLE/PIB in patients with renal impairment Design No randomisation Open label W8 W12 W16 18 years, HCV genotype 1-6 HCV RNA > 1 000 IU/mL Treatment-na ve (TN) or treatment- experienced (TE) without DAA except SOF With or without cirrhosis Chronic kidney disease stage 3b, 4 or 5 * No HBV or HIV co-infection N = 84 GLE/PIB N = 13 GLE/PIB N = 4 GLE/PIB 8W: no cirrhosis, TN (GT 1 to 6) or TE (GT 1,2, 4,-6 12W: cirrhosis, TN (GT 1 to 6) or TE (GT 1,2, 4,-6) 16W: TE, GT3 * stage 3b: eGFR 30 to < 45 mL/min/1.73m ; stage 4: eGFR 15 to < 30 mL ; stage 5: eGFR<15 mL or dialysis-dependent GLE/PIB: 100/40 mg 3 tablets QD, administered regardless of timing of dialysis (hemo- or peritoneal) Primary endpoint SVR12(HCV < LLOQ) EXPEDITION-V Gane E. N Engl J Med. 2017; 377:1448-55
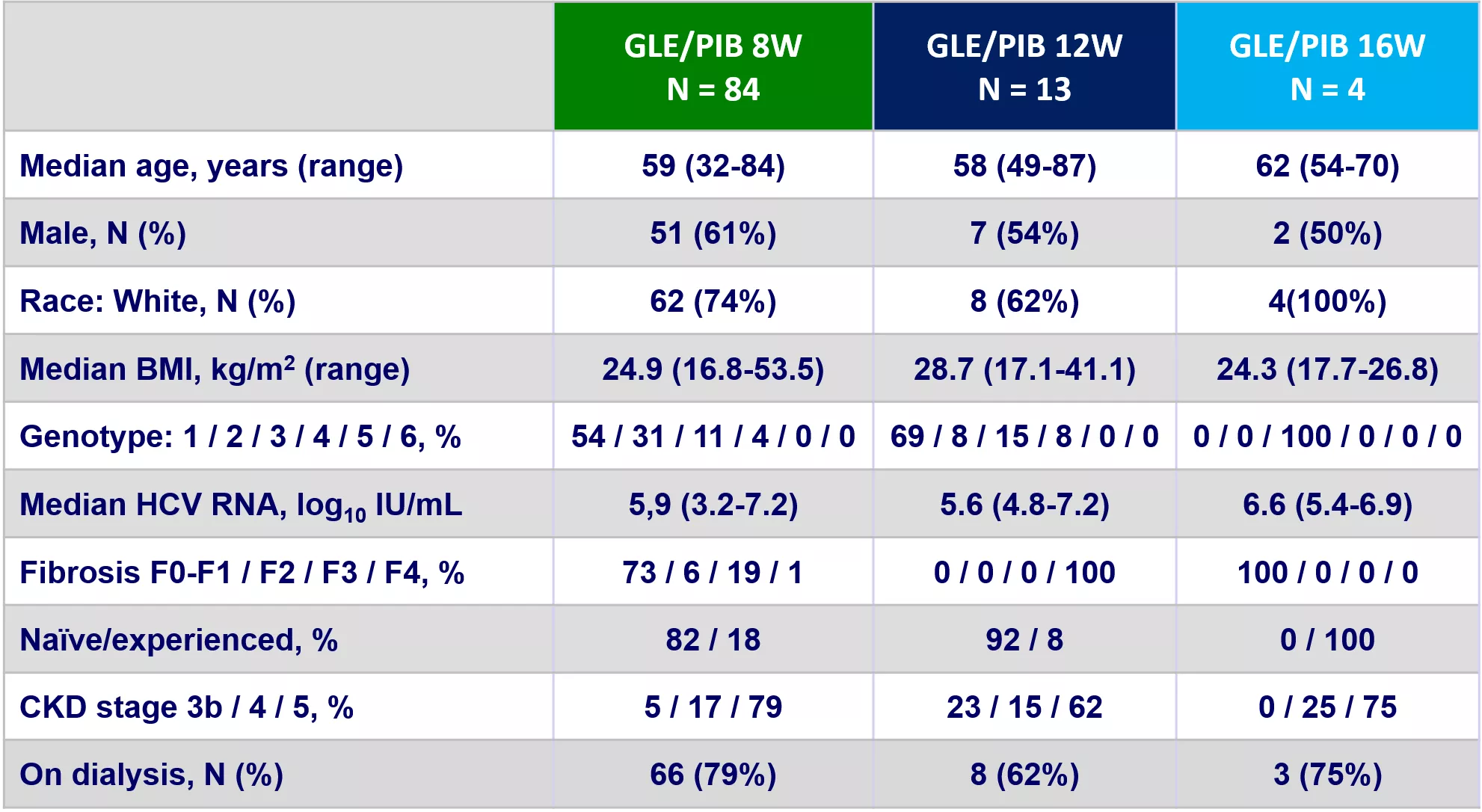
EXPEDITION-V Study: GLE/PIB in patients with renal impairment Baseline characteristics GLE/PIB 8W N = 84 GLE/PIB 12W N = 13 GLE/PIB 16W N = 4 Median age, years (range) 59 (32-84) 58 (49-87) 62 (54-70) Male, N (%) 51 (61%) 7 (54%) 2 (50%) Race: White, N (%) 62 (74%) 8 (62%) 4(100%) Median BMI, kg/m2(range) 24.9 (16.8-53.5) 28.7 (17.1-41.1) 24.3 (17.7-26.8) Genotype: 1 / 2 / 3 / 4 / 5 / 6, % 54 / 31 / 11 / 4 / 0 / 0 69 / 8 / 15 / 8 / 0 / 0 0 / 0 / 100 / 0 / 0 / 0 Median HCV RNA, log10IU/mL 5,9 (3.2-7.2) 5.6 (4.8-7.2) 6.6 (5.4-6.9) Fibrosis F0-F1 / F2 / F3 / F4, % 73 / 6 / 19 / 1 0 / 0 / 0 / 100 100 / 0 / 0 / 0 Na ve/experienced, % 82 / 18 92 / 8 0 / 100 CKD stage 3b / 4 / 5, % 5 / 17 / 79 23 / 15 / 62 0 / 25 / 75 On dialysis, N (%) 66 (79%) 8 (62%) 3 (75%) EXPEDITION-V Gane E. N Engl J Med. 2017; 377:1448-55
EXPEDITION-V Study: GLE/PIB in patients with renal impairment Primary Endpoint (SVR12) % 100 100 ** 100 97 96 * 100 80 60 40 20 N = 84 13 4 101 98 0 GLE/PIB 8W GLE/PIB 12W GLE/PIB 16W Overall ITT Overall mITT * 1 patient had missing data and 2 patients discontinued treatment ** 1 patient with NS5A Y93H RAS achieved SVR12 EXPEDITION-V Gane E. N Engl J Med. 2017; 377:1448-55
EXPEDITION-V Study: GLE/PIB in patients with renal impairment Adverse events and laboratory abnormalities, % Overall N = 101 55 13 / 12 * 2 Any adverse event Grade 3 adverse event / serious adverse event Adverse event leading to discontinuation Adverse events in > 5% of patients Pruritus Hypertension Generalized pruritus Bronchitis Diarrhea 22 22 12 12 10 Laboratory abnormalities AST grade 3 (5 x ULN) ALT grade 3 (5 x ULN) Total bilirubin grade 3 (> 3 x ULN) 0 0 0 * No AE related to treatment No death observed EXPEDITION-V Gane E. N Engl J Med. 2017; 377:1448-55
EXPEDITION-V Study: GLE/PIB in patients with renal impairment Renal function Of the 24 patients with CKD stage 3b or 4 and with available results, eGFR remained unchanged from screening to end of treatment and post-treatment week 4: 27.1 9.2 vs 26.4 9.8 vs 27.4 11.6 mL/min/1.73m CKD stage remained unchanged in 22/24 patients with end of treatment results and declined in 2/24 from screening to end of treatment EXPEDITION-V Gane E. N Engl J Med. 2017; 377:1448-55
EXPEDITION-V Study: GLE/PIB in patients with renal impairment Summary GLE/PIB is highly efficacious in patients with chronic kidney disease stage 3b to 5 with the label recommended treatment durations based on genotype, cirrhosis status and prior treatment experience Treatment was well-tolerated Overall, renal function remained unchanged after treatment in pre-dialysis patients assessed EXPEDITION-V Gane E. N Engl J Med. 2017; 377:1448-55