OSHA Bloodborne Pathogens Training and Hepatitis B Virus
Covering the basics of OSHA Bloodborne Pathogens Standard, this training material provides essential information on bloodborne pathogens, including Hepatitis B Virus (HBV). It emphasizes the importance of training for individuals with occupational exposure, outlining crucial topics such as Hepatitis B vaccination, emergency procedures, exposure incidents, and more. The content also explains the definition of bloodborne pathogens, focusing on pathogens like HBV and HIV. Additionally, it delves into the details of Hepatitis B Virus, its epidemiology, symptoms, transmission, and impact on health. Overall, this training resource serves as a comprehensive guide for academic programs and workplaces aiming to educate individuals on bloodborne pathogens and Hepatitis B Virus.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
OSHA BLOODBORNE PATHOGENS
DISCLAIMER The following slides cover the basic components of the OSHA Bloodborne Pathogens Standard, 29 CFR 1910.1030. These slides can be used as a reference for USC academic programs that provide bloodborne pathogens awareness training to their non-paid students. Additional guidance on bloodborne pathogens training for academic programs is posted on USC s Biological Safety Training webpage. Academic programs that place students in work environments where the student may have an occupational exposure to bloodborne pathogens are responsible for providing training to these students. Bloodborne pathogens awareness training will be most effective if these slides are customized to apply to the workplace that the training will address. Each academic program should maintain training records for students upon completion of training.
TRAINING TOPICS ALL EMPLOYEES WITH OCCUPATIONAL EXPOSURE TO BBP RECEIVE ANNUAL TRAINING I. II. III. IV. V. VI. VII. Hepatitis B Vaccination VIII. Emergencies Involving Blood or OPIM IX. Procedure for Exposure Incidents X. Post-Exposure Evaluation and Follow-up XI. Housekeeping, Laundry, Signs and Labels XII. Recordkeeping XIII. Questions and Answers Bloodborne Pathogen Diseases OSHA Bloodborne Pathogen Standard Exposure Control Plan Recognition of Exposure Incident Engineering Controls and Work Practices Personal Protective Equipment
IMPORTANT DEFINITION Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and can cause disease in humans. These pathogens include, but are not limited to, HBV (hepatitis B virus), and HIV (human immunodeficiency virus).
HEPATITIS B VIRUS Hepatitis means inflammation of the liver. The liver is a vital organ. Hepatitis B can be a serious liver disease from infection with the hepatitis B virus. Acute Hepatitis B: short-term infection Occurs within 6 months Range in severity: no symptoms to serious condition Many adults can clear the virus without treatment Chronic Hepatitis B: lifelong HBV infection ~5% of infected adults develop chronic HBV. Chronic HBV can cause serious health problems
HEPATITIS B VIRUS Epidemiology Many people don t know they are infected. Rates of acute infection have declined ~82% since 1991. Estimated 880,000 to 1.89 million people have chronic hepatitis B. Symptoms Many people have no symptoms. Possible symptoms include: fever, feeling tired, not wanting to eat, upset stomach, dark urine, grey stool, joint pain, and yellow skin and eyes. Transmission HBV virus is spread when blood or body fluids from an infected person enters the body of an uninfected person. Percutaneous injury (e.g. needlestick or laceration) Contact with a mucous membrane or non-intact skin Estimated risk for occupational percutaneous HBV infection is 6-30%
HEPATITIS C VIRUS Epidemiology For some people, HCV is a short-term illness but for 70% 85% infected with HCV, it becomes a long-term infection. Symptoms Chronic Hepatitis C is a serious disease that can result in long-term health problems. Majority of infected persons may not be aware of their infection (not clinically ill). Transmission HCV is a blood-borne virus. Estimated risk for infection after needlestick or cut exposure to HCV-infected blood is ~1.8% There is no vaccine for Hepatitis C.
HUMAN IMMUNODEFICIENCY VIRUS Epidemiology In 2021, 36,136 people diagnosed with HIV. Annual new diagnoses declined 7% (2017 2021). Symptoms Weakens a person s immune system by destroying important cells that fight disease and infection. No effective cure, but with proper medical care, HIV can be controlled. Transmission Risk of workers exposed to HIV on the job is very low. Main risk of HIV transmission is being stuck with HIV-contaminated needle or other sharps. Estimated risk of HIV infection from being stuck with a needle used on an HIV-infected person is less than 1%.
IMPORTANT DEFINITIONS Blood means human blood, human blood components, and products from human blood. Other Potentially Infectious Materials (OPIM): 1) Body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, any body fluid that is visibly contaminated with blood 2) Any unfixed human tissue or organ 3) HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV- containing cultures or solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV
IMPORTANT DEFINITION Universal Precautions means all human blood and certain human body fluids are treated as if known to be infectious for HIV, HBV, HCV, and other bloodborne pathogens.
OCCUPATIONAL SAFETY AND HEALTH ADMINISTRATION OSHA s Mission save lives, prevent injuries and protect health of workers BBP Standard protects employees from exposure to blood or OPIM. 29 CFR 1910.1030 Copy online at www.osha.gov
IMPORTANT DEFINITIONS Occupational Exposure means reasonably anticipated skin, eye, mucous membrane, or parenteral contact with blood or other potentially infectious materials that may result from the performance of an employee's duties. Parenteral means piercing mucous membranes or the skin barrier through such events as needlesticks, human bites, cuts, and abrasions.
EXPOSURE CONTROL PLAN (ECP) ECP is provided to eliminate or minimize occupational exposure to bloodborne pathogens ECP template available on Environmental Health and Safety website on the Biological Safety page Template must be tailored to be specific for your work area or department
ECP CONTENTS Determination of employee exposure Implementation of exposure control methods Universal precautions Engineering and work practice controls Personal protective equipment Housekeeping Hepatitis B vaccination Post-exposure evaluation and follow-up Procedures for evaluating exposure incidents Communication of hazards and training Recordkeeping
ECP AVAILABILITY & REVIEW Written (site-specific) plan must be accessible to all employees Employees must be provided a free copy within 15 days if requested Must be reviewed and updated: At least annually More frequently if necessary to reflect new or modified tasks or positions that affect exposure
EXPOSURE DETERMINATION List of job classifications in which all or some employees at an institution have occupational exposure. May include a list of tasks and procedures in which occupational exposure may occur Part-time, temporary, contract and per diem employees are covered by the BBP standard.
PROGRAM ADMINISTRATION Site-specific ECP should document who is responsible for the following duties: Implementation of the ECP Providing and maintaining PPE, engineering controls, labels and red bags Ensuring all medical actions are performed, and employee health and OSHA records maintained Training, documentation of training, and making the ECP available to employees Employees must comply with procedures and work practices outlined in the ECP
IMPORTANT DEFINITIONS Engineering Controls means controls that isolate or remove the bloodborne pathogens hazard from the workplace. Work Practice Controls means controls that reduce the likelihood of exposure by altering the manner in which a task is performed.
ENGINEERING CONTROLS & WORK PRACTICE CONTROLS Used to prevent or minimize exposure to bloodborne pathogens Specific controls described in ECP Examine, maintain or replace engineering controls regularly to ensure effectiveness
HANDWASHING Handwashing facilities readily accessible to employees When not feasible, provide antiseptic hand cleaner with cloth/paper towels or antiseptic towelettes Wash hands and other skin with soap and water or flush mucous membranes with water following contact with blood or OPIM
CONTAMINATED SHARPS Contaminated needles or other sharps shall not be bent, recapped, or removed, unless the user can demonstrate no alternative is feasible or action is required by a procedure Such activities must be approved by a safety professional and accomplished using a mechanical device or one-handed technique Shearing or breaking needles is prohibited Contaminated sharps shall immediately be placed in containers containers shall be: Puncture-resistant, labeled or color-coded, and leak-proof on sides and bottom
WORK PRACTICES & PROCEDURES Eating, drinking, smoking, applying cosmetics or lip balm, and handling contact lenses are prohibited in work areas where occupational exposure may occur Food and drink not kept where blood or OPIM are present Procedures involving blood or OPIM performed to minimize splashing, spraying, spattering, and droplets Mouth pipetting/suctioning of blood or OPIM is prohibited
CONTAINERS & EQUIPMENT Place specimens of blood or OPIM in a container that prevents leakage If outside contamination of primary container, place it in second container that prevents leakage Second container should be red or have biohazard label If specimen could puncture primary container, place it in puncture-resistant secondary container Examine equipment that may be contaminated prior to servicing or shipping Decontaminate equipment as necessary
ENGINEERING CONTROLS & WORK PRACTICE CONTROLS Identify need for changes in controls Evaluate new procedures/products Involve front-line works and management in evaluation process
IMPORTANT DEFINITION Personal Protective Equipment is specialized clothing or equipment worn by an employee for protection against a hazard. General work clothes not intended to function as protection against a hazard are not considered to be personal protective equipment.
PERSONAL PROTECTIVE EQUIPMENT (PPE) Provided at no cost to employees Training in proper PPE for tasks ECP describes PPE available ECP describes location of PPE and who makes PPE available
PERSONAL PROTECTIVE EQUIPMENT (PPE) Wash hands immediately after removing gloves or other PPE Remove PPE when contaminated and before leaving work area Remove in such a way as to avoid contact with the outer surface ECP describes proper PPE disposal
PPE: GLOVES Wear gloves when there may be hand contact with blood or OPIM, and when touching contaminated items or surfaces Replace gloves if torn, punctured or contaminated, or if compromised Never wash or decontaminate disposable gloves for reuse
PPE: UTILITY GLOVES, FACE AND EYE PROTECTION Utility gloves - may be decontaminated for reuse if not compromised; discard if signs of cracking, peeling, tearing, puncturing, or deterioration Face and eye protection wear when splashes, sprays, spatters, or droplets of blood or OPIM pose a hazard to eyes, nose, or mouth
HEPATITIS B VACCINATION Efficacy Safe & effective to prevent infection Safety No known serious side effects Most have no side effects, but mild side effects can occur (e.g. low fever or sore arm from shot) Method of administration HBV vaccine given as 3 shot series over 6 months Entire series needed for long-term protection Benefits HBV vaccine is the best protection
HEPATITIS B VACCINATION HBV vaccine series is available at no cost after initial training and within 10 days of assignment to all employees with occupational exposure Vaccination is encouraged unless: 1) Documentation employee previously received the series 2) Antibody testing reveals immunity 3) Medical evaluation shows contraindicated
HEPATITIS B VACCINATION If an employee declines vaccination, they must sign a declination form Employees who decline may request to be vaccinated at a later date at no cost Documentation must be kept of refusal of vaccination (location defined in ECP)
HEPATITIS B VACCINATION Location where vaccination is provided is defined in ECP (may vary by campus) Columbia campus: Student Health Services Health care professional s written opinion provided to employee within 15 days of completing the evaluation. Limited to whether employee requires HBV vaccine and if vaccine was administered
IMPORTANT DEFINITION Exposure Incident means a specific eye, mouth, other mucous membrane, non-intact skin, or parenteral contact with blood or other potentially infectious materials that results from the performance of an employee's duties.
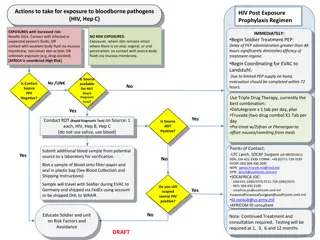
POST-EXPOSURE FIRST AID & MEDICAL EVALUATION ECP describes who to contact if an exposure occurs Follow initial first aid procedures Clean wound, flush eyes, etc. A confidential medical evaluation and follow-up will be conducted
POST-EXPOSURE EVALUATION & FOLLOW-UP Document routes of exposure Identify and document source individual Obtain consent and make arrangements to have source individual tested to determine HIV, HCV & HBV infectivity Convey test results to healthcare provider No testing required if source is already known to be HIV, HCV or HBV positive
POST-EXPOSURE EVALUATION & FOLLOW-UP (CONTINUED) Assure exposed employee is provided source individual s test results After consent, collect exposed employee s blood test HBV & HIV serological status If employee does not give consent for HIV testing during initial blood collection, preserve baseline sample for at least 90 days Perform testing if exposed employee elects to have sample tested during waiting period
ADMINISTRATION OF POST-EXPOSURE EVALUATION & FOLLOW-UP Healthcare professional(s) responsible for employee s HBV vaccination & post-exposure evaluation are given copy of BBP standard Health care professional evaluating an exposure incident receives the following: Description of employee s relevant job duties Route(s) of exposure Circumstances of exposure If possible, results of source blood test Relevant employee medical records (e.g. vaccinations) Employee is provided copy of health care professional s written opinion within 15 days
PROCEDURES FOR EVALUATING THE CIRCUMSTANCES SURROUNDING AN EXPOSURE INCIDENT Circumstances of all exposure incidents will be reviewed to determine: Engineering controls used at the time Work practices followed Description of device used (type & brand) PPE or clothing used at time of exposure Location of incident Procedure performed when incident occurred Employee s training Percutaneous injuries from contaminated sharps will be recorded in Sharps Injury Log If necessary, make revisions to the ECP
HOUSEKEEPING Regulated waste is placed in closable containers constructed to contain contents Labeled or color-coded & closed prior to removal Contaminated sharps are discarded in closable, puncture- resistant, leak proof containers that are labeled or color- coded Bins and pails are cleaned & decontaminated Broken glassware is only picked up using a mechanical device
LAUNDRY ECP describes who does laundering Laundering requirements must be met: Handle contaminated laundry as little as possible Place wet contaminated laundry in leak-proof, labeled or color-coded containers before transport ECP indicates appropriate PPE when handling/sorting contaminated laundry
SIGNS & LABELS Warning labels affixed to regulated waste containers, refrigerators and freezers containing blood or OPIM; and other containers used to store or transport blood or OPIM Labels include biohazard symbol Labels affixed close to container to prevent loss or unintentional removal Red bags/containers may be substituted for labels Exemptions to labeling requirements may apply Blood released for transfusion, individual containers placed in labeled containers, regulated waste after decontamination