Nuclear Chemistry: The Formation of New Elements
Delve into the fascinating world of nuclear chemistry with a focus on the formation of new elements through processes like radioactive decay, nuclear fusion, and nuclear fission. Understand how nuclear reactions change the identity of elements and explore the dynamics of nuclear chain reactions. Gain insights into the mechanisms behind the creation of new elements through balanced nuclear equations and the interplay of protons and neutrons within atomic nuclei.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Living By Chemistry SECOND EDITION Unit 1: ALCHEMY Matter, Atomic Structure, and Bonding
Lesson 16: Old Gold Formation of Elements
ChemCatalyst 1. What patterns do you notice in the fusion reactions? 2. Do you think gold can be created on Earth by a fusion reaction? Explain your thinking.
Key Question How are new elements formed?
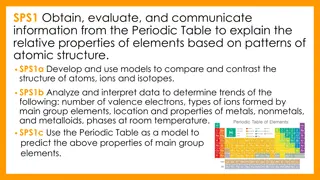
You will be able to: explain how different elements are formed through nuclear reactions write a balanced nuclear equation describe the mechanism behind a nuclear chain reaction
Prepare for the Activity Work individually. You will need a copy of the periodic table and the isotope chart from Lesson 14.
Discussion Notes Nuclear processes can be written as nuclear equations. During alpha decay, the nucleus of an atom emits a helium nucleus, transforming the element into an element with a smaller nucleus. During beta decay, a neutron inside the nucleus of an atom emits an electron.
Discussion Notes (cont.) Nuclear fusion involves the joining together of nuclei. Fission involves a nucleus breaking up into smaller nuclei. Nuclear reactions change the identity of an element. Nuclear fusion produces bigger (heavier) elements from smaller (lighter) ones.
Discussion Notes (cont.) Nuclear Chain Reactions
Discussion Notes (cont.) Nuclear fission is a process that releases enormous amounts of energy. Nuclear fission can result in a nuclear chain reaction that produces a great deal of energy.
Wrap Up How are new elements formed? Radioactive decay, nuclear fusion, and nuclear fission are all nuclear processes that result in the creation of new elements. The mass of a nucleus changes when neutrons or protons are added or lost. The identity of an element changes when its nucleus gains or loses protons.
Wrap Up (cont.) Radioactive decay happens in the natural world around us. Fission can be spontaneous for unstable nuclei, or it can be provoked using nuclear bombardment and other methods. Fusion of nuclei to form different isotopes happens in the stars.
Check-In In a paragraph, defend this statement: If you want to find gold, your best bet is to dig old gold out of the ground. Your chances of making new gold are slim.