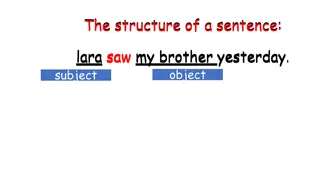
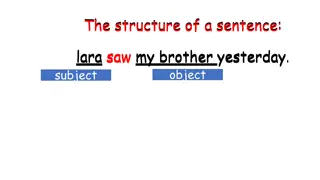
Human Subject Research
This content discusses various categories of human subject research, including exempt categories and clinical trials focusing on interventions and outcomes. It also touches upon research involving identifiable data and private information.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Human Subject Research Audrey Williams, PhD Audrey Williams, PhD
Human Subjects Living subject Obtaining data through intervention or interaction Identifiable data Private information o Reasonably expects no observation/recording o Reasonably expects not be made public
Clinical Trial Human subject research Prospectively assigned to intervention o Drug, device, procedure, delivery system (including interviews or telemedicine), etc. o Strategies to change health behavior (diet, cognitive therapy, exercise, etc.) Health related or behavioral outcome o Mood, reading comprehension, biological parameters, quality of life
Exempt Categories Category 1 educational setting with normal education practices Category 2 Educational tests, surveys, interviews unless identifiable AND AND poses a risk o Cannot include children Category 4 Data or samples previously collected with no identifiable data o Existing at time of submission* o Cannot be coded with a link to identifiers
Exempt Categories Category 3 surveys of public officials (such as politicians) Category 5 Public benefit of service programs Category 6 Taste and food quality evaluation New Common Rule 1/21/2019 o Category 7 Storage or maintenance of samples or data for which broad consent is required o Category 8 Secondary research for which broad consent is required