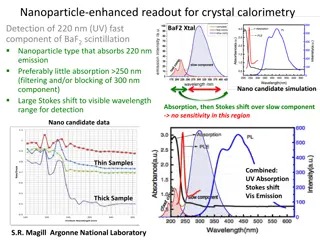
HEAT AND CALORIMETRY
Covering the fundamentals of heat transfer, specific heat, and calorimetry, this informative content explains the units to measure heat flow, the concept of specific heat, and how to calculate changes in thermal energy. Discover why water has a high specific heat capacity and learn about the tools used, like calorimeters, in heat measurement experiments. Gain insights into converting between calories and joules, and explore examples showcasing different specific heat values for various materials. Delve into the world of heat and energy transformations with this comprehensive guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
HEAT AND CALORIMETRY
HEAT Represented by q Energy that transfers from one object to another because of a temperature difference between them Heat always flows from a warmer object to a cooler object
UNITS TO MEASURE HEAT FLOW 2 ways the calorie and the joule calorie (cal) the quantity of heat needed to raise the temperature of 1 g of pure water 1oC Always written with a small c (except with dietary calories) Dietary Calorie is always written with a capital C 1 Dietary calorie is equal to one kilocalorie, or 1000 calories 1 Calorie = 1 kilocalorie = 1000 calories
JOULE SI Unit of Energy (J) One joule of heat raises the temp of 1 g of pure water 0.2390oC
CONVERTING BETWEEN CALORIES AND JOULES 1 J = 0.2390 cal 4.184 J = 1 cal
SPECIFIC HEAT Represented by C Amount of heat required to raise the temperature of 1 g of the substance 1oC This is why some things heat up more quickly than others
EXAMPLE Cwater= 4184 J/kg C Csand= 664 J/kgC This is why land heats up quickly during the day and cools quickly at night and why water takes longer
WHY DOES WATER HAVE SUCH A HIGH SPECIFIC HEAT? Water molecules form strong bonds with each other; therefore it takes more heat energy to break them Metals have weak bonds and do not need as much energy to break them
HOW TO CALCULATE CHANGES IN THERMAL ENERGY Q = mC T Q = change in thermal energy m = mass of substance T = change in temperature (Tf Ti) Cp = specific heat of substance
CALORIMETER This is used to help measure the amount of heat involved in a chemical reaction

 undefined
undefined