Emerging Technologies in Alternative Prime Movers and Fuel Cells
Explore the world of unconventional hybrid prime movers, fuel cells, and low-to-zero emission vehicles through advanced technologies such as green hydrogen production systems, fuel cell electric vehicles (FCEVs), and more. Discover the potential of these innovative systems in revolutionizing transportation towards sustainability and efficiency.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Unconventional Hybrid Prime Movers P M V Subbarao Professor Mechanical Engineering Department An Attempt to Use A Pair of Most reversible Prime Movers.....
Future Conceptual Hybrid Scheme High Energy density energy source Conventional: Fossil Fuels Digestive Prime Mover Conventional: CI & SI I C Engines Unconventional: Green Fuels Unconventional: HCCI, RCCI I C Engines & Fuel Cells Conventional: Electric Battery Conventional: Electric MGs Unconventional: Hydraulic MGs Unconventional: Hydraulic storage Low Energy density energy source Tractive Prime Mover
Low to Zero Emission Vehicles Various Technologies for Automobiles Vehicle weight challenges 2nd generation biofuels high Fuel cell vehicles transportation Long range public transport Public transport Economy of fuel Plug-in hybrids City LDVs Everyday use Everyday use Battery vehicle Economy of propulsion system 2nd car Energy density acceptance Electro cycle safety distance low Long trips (highway)
A Fuel Cell Electric Vehicle (FCEV) A fuel cell electric vehicle (FCEV) is a type of electric vehicle that uses a fuel cell and an electric motor as its propulsion system. The onboard fuel cell directly converts chemical energy to electric energy. A hydrogen fuel cell is the most popular type that has been used in fuel cell vehicles. FCEVs are targeted to provide customers with the benefits of battery electric vehicles such as low to zero emission, high performance, and low maintenance, without compromising range and refill time. In the past fifty years, over a hundred FCEV models have been developed.
Timeline of major events in fuel cell vehicle developments Sir William Grove in 1839 Sir William Grove in 1839
A Hydrogen Fuel Cell A hydrogen fuel cell is the most popular type that has been used in fuel cell vehicles. It consumes hydrogen and oxygen and only produces water vapor and heat as exhaust products. Therefore a hydrogen fuel cell vehicle produces zero tailpipe greenhouse gas (GHG) emission. In general, hydrogen is produced using either steam methane reforming or electrolysis of water. The adoption of FCEVs would significantly reduce a country s dependence on foreign oil.
Hydrogen: The Primordial Bestower to The Ecosystem On the ecosystem scale, hydrogen supports microbial communities in most terrestrial, aquatic, and host-associated ecosystems. This is the primordial electron donor. In all biological systems, hydrogen metabolism is the most superior energy generator. Better understanding of hydrogen metabolism is the backbone of future renewable energy. A mole of electrons is 6.022 1023 electrons. The charge (F) is N e, where e is 1.602 10 19 C. F = N e = 96485 C
The Atomistic Hydrogen Metabolism Most irreversible metabolism is combustion of hydrogen. + 2 ( ) ( ) 2 + ( ) H gas O gas H O liquid 2 2 2 286 / ( ) MJ kmol ThermalEne rgy Hydrogen gas forms explosive mixtures with air in concentrations from 4 74%. The explosive reactions may be triggered by spark, heat, or sunlight. A controlled Combustion is used in Next generation Hydrogen DI- SI I.C. Engines.
The Holistic Art of Hydrogen Metabolism Microorganisms conserve energy by metabolizing hydrogen. Oxidation of this high-energy fuel yields electrons that can be used for respiration and carbon-fixation. Electrochemical reaction of Hydrogen is the most reversible reaction. Electrochemical reaction produces electricity At cathode O2 + 2e- O2- At anode H2 + O2- H2O + 2e- Overall reaction H2+ O2 H2O It is the change in this Gibbs free energy of formation, Gf, that generates electrical energy. A fuel cell only can convert this change in Gibbs free energy into electrical energy. = G G G products reactants f f f
Change in Gibbs Free Energy If the fuel cell is a reversible device, then all this Gibbs free energy is converted into electrical energy.
Electrical Energy Generated by An Ideal Fuel Cell In the hydrogen fuel cell, two electrons pass round the external circuit for each molecule of hydrogen consumed. Therefore, for one mole of hydrogen used, 2N electrons pass round the external circuit where N is Avogadro s number. If e is the charge on one electron, then the charge that flows is Ne 2 2 = If E is the voltage of the fuel cell, then the electrical work done moving this charge round the circuit is Electrical work done = charge voltage = -2F.V Joules. Columbs F
Ideal Cell Voltage If the system is reversible (or has no losses), then this electrical work done will be equal to the Gibbs free energy released. gf = 2 V . F g f = V 2 F This fundamental equation gives the electromotive force (EMF) or reversible open circuit voltage of the hydrogen fuel cell. In practice the voltage would be lower than this because of the voltage drops in the circuit elements. Some of the irreversibilities apply a little even when no current is drawn, so even the OCV of a fuel cell will usually be lower.
Ideal Fuel Cell Capacity The ideal efficiency of a reversible galvanic cell is related to the enthalpy for the cell reaction by G S = = 1 T id H H idwill be 100% if the electrochemical reaction involves no change in the number of gas moles, that is, when S is zero. 237 1 . G = = = . 0 83 id H 285 8 . H 2
The Fundamental Law : Electrochemical Reaction In a fuel system, generating chemical energy into electrical energy can be done by transforming hydrogen and oxygen into steam were restricted by efficiency limits of the Carnot thermal cycle. (Larminie and Dicks 2000) Tamb carnot = 1 T max
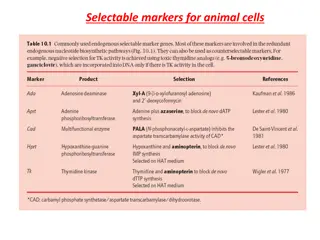
Fuel Cell Technologies There are six major types of fuel cells depending on the type of their electrolyte. Proton exchange membrane (PEM) or Polymer exchange membrane fuel cells (PEMFCs) Alkaline fuel cells (AFCs) Phosphoric acid fuel cells (PAFCs) Molten carbonate fuel cells (MCFCs), Solid oxide fuel cells (SOFCs) Direct methanol fuel cells (DMFCs)
Assignment 3 Select any one of fuel cell from following list: Proton exchange membrane (PEM) or Polymer exchange membrane fuel cells (PEMFCs) Alkaline fuel cells (AFCs) Phosphoric acid fuel cells (PAFCs) Molten carbonate fuel cells (MCFCs), Solid oxide fuel cells (SOFCs) Direct methanol fuel cells (DMFCs) Write a scientific report on mathematical modelling and analysis of thermodynamic performance of the selected fuel cell. Last date of submission: 13 November 2022.
Classification based on electrolyte used Max attained production V e H2 O2 AFC 600C 100 kW OH - H2O O2 600C 250 kW H2O O2 PEMFC H2 H + CH3OH DMFC H + 800C 2 MW H2O CO O2 2 H + PAFC 1900C 11 MW H2O H2 H2 O2 6500C 2 MW MCFC CO32- H2O H2 CO2 SOFC 10000C 1 MW O2 O 2- H2O Fuel Oxygen Anode Cathode Electrolyte
Fuel Consumption in A Hydrogen FC For every molecule of hydrogen (H2), two electrons are Liberated. MH2 = 1.04445 * 10 -5 kg H2 / s - kA For 60 kW plant : I = Power / Voltage = 60000 / 0.7 = 85 kA = 5 89 5 . 10 / mH kg s Mass flow rate of hydrogen : 2 Number of cells in a stack Therefore, for 60 kW, Total current = 60000 / 0.7 = 85000 amps Required area of the cell = Total current / Current density No. of cells = required area / area of the individual cell
Limit on Size of A Single Cell : Limited by Irreversibilities Actual potential of the cell is less than the equilibrium potential due to irreversible losses or polarization. Losses or polarizations limits Actual Performance Activation over potential ( Vact) Flow of ions should overcome the electronic barrier. Ohmic over potential ( Vohm) Resistance offered by the total cell components to the flow. Concentration over potential ( Vcon) Gas transport losses, dilution of fuel as the reactions progress. V-I Characteristics of the Fuel cell
Performance characteristics of a single SOFC Cell 1.0E+00 3.5E+03 3.0E+03 8.0E-01 Power density (W m-2) Cell potential (volts) 2.5E+03 6.0E-01 2.0E+03 1.5E+03 4.0E-01 1.0E+03 2.0E-01 5.0E+02 0.0E+00 0.0E+00 0 2000 4000 6000 8000 Current density (A m-2)
Performance characteristics of a single SOFC Cell 1.0E+00 3.5E+03 3.0E+03 8.0E-01 Power density (W m-2) Cell potential (volts) 2.5E+03 6.0E-01 2.0E+03 1.5E+03 4.0E-01 1.0E+03 3000 2.0E-01 2500 5.0E+02 Power density (W m-2) 2000 0.0E+00 0.0E+00 1500 0 2000 4000 6000 1000 8000 Current density (A m-2) 500 0 1400 1.4 1200 1.2 1 1000 0.8 0.6 800 0.4 Operating temperature (K) Cell voltage (volt)
The influence of temperature on the operating voltage of different fuel cells