Compounds of Silver
Delve into the various compounds of silver discussed in the course by Dr. Atul Kumar Singh. Explore reactions with different substances like NaOH, KCN, sodium thiosulphate, iodine, and base metals. Discover complex formations, displacements, and transformations involving silver. Unveil the unique properties and chemical interactions of silver compounds, shedding light on their significance in chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Compounds of Silver Course Instructor: Dr. Atul Kumar Singh Assistant Professor Department of Chemistry M. L. Arya College, Kasba Purnia -854330 India
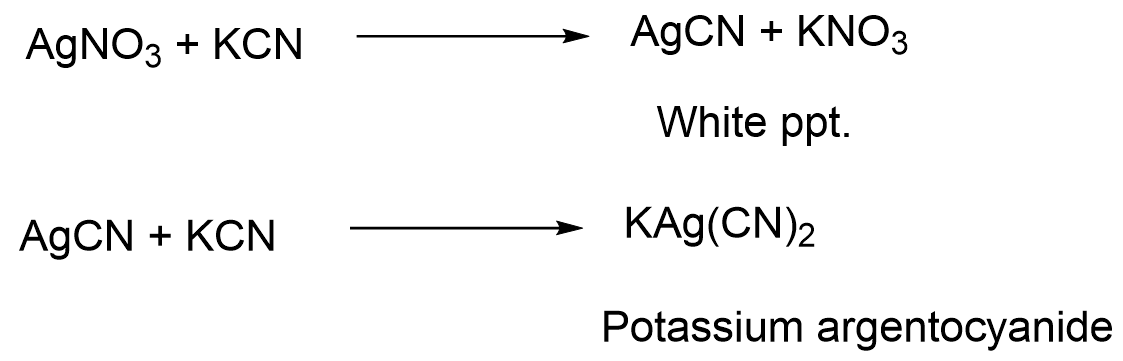
Reaction with a solution of NaoH Ag2O is originally brown in color but turn black when dried . Reaction with KCN AgCN dissolve in excess of KCN forming a complex, Potassium argentocyanide
. Reaction with Sodium thiosulphate Ag2S2O3 dissolve in excess of Sodium thiosulphate forming a complex, Sodium argentothiosulphate
. Reaction with iodine . Reaction with Base metals Silver is displaced from an aqueous solution of silver nitrate
. Reaction with phosphine, arsine and stibine Reaction with NH4OH
The ammonical solution of silver nitrate gives the following reations Reaction with acetylene it converts glucose to gluconic acid it oxidises formaldehyde to formic acid