Bomb Calorimetry
Bomb calorimetry is a technique used to measure the heat of combustion for organic materials. By saturating the material with oxygen in a sealed container and igniting it, the energy produced can be accurately quantified. This method finds applications in assessing energy content in food, safety testing in pharmaceuticals, and determining explosive potential. The components, functioning, and formula of a bomb calorimeter are essential in understanding its role in heat measurement and analysis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
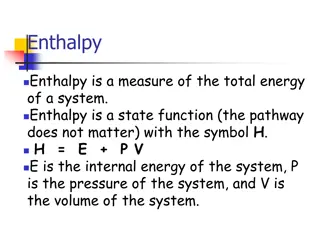
Introduction Introduction Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The principle of operation is to saturate the material with oxygen, within a sealed container (the bomb) and ignite using a hot wire. During the rapid combustion carbon molecules are converted to carbon dioxide, hydrogen to water and nitrogen to gaseous nitrogen. The recorded enthalpy change is thus a sum of all bonds broken and bonds made converting the organic solid into simple gaseous molecules. The measured enthalpy change for this type of analysis is often referred to as the enthalpy of formation. Note that, in fact, this type of calorimeter operates at constant volume. As such, the energy measured is internal energy change ( U) and not enthalpy change ( H).
With the help of a bomb calorimeter, the actual amount of energy produced by food if oxidized (burned) completely can be measured. Within the pharmaceutical industry, bomb calorimetry is used mainly for safety testing of materials. From a calculation of U an assessment can be made for the explosive force that could be produced if the material was to detonate during rough treatment. During a safety test, the material is shocked under varying conditions of temperature and relative humidity to determine if detonation would take place.
Bomb Calorimeter Bomb Calorimeter Diagram Diagram The general parts of a bomb calorimeter consists of: Metal container with an insulated jacket Steel bomb Stirrer Thermometer Reactant gas supply Ignition unit Water
Bomb Calorimeter Parts and Bomb Calorimeter Parts and Function Function The reaction of combustion takes place in the bomb, a sealed heavy-walled container. The bomb is filled with a reactant gas such as oxygen through a supply connection and valve. The sample is placed in a platinum crucible and the ignition circuit connected to the bomb will start the reaction. The bomb is situated in the calorimeter, which is a closed metal can containing water that is constantly stirred to maintain a uniform temperature. The water surrounding the bomb absorbs the heat generated from the reaction, and a thermometer records the changes in the temperature of the water. The calorimeter is insulated by a jacket, which prevents heat loss to the surroundings.
Bomb Calorimeter Bomb Calorimeter Formula Formula The amount of heat (Q) transferred to or from an object can be calculated using the formula: Where m is the mass of the object, C is the specific heat of the the object, and T is the change in temperature of the object. The change in temperature is calculated as Tf (final temperature) - Ti (initial temperature), and can be positive or negative, depending on the respective temperatures. The specific heat (C) of a substance (also known as the heat capacity) is the amount of heat needed to increase the temperature of the substance by 1 C.




![❤Book⚡[PDF]✔ Fool Me Once: Should I Take Back My Cheating Husband?](/thumb/20473/book-pdf-fool-me-once-should-i-take-back-my-cheating-husband.jpg)