Biothermodynamics: Course Overview and Curriculum Details
Dive into the world of biothermodynamics with a detailed look at core courses, textbooks, and the history of the course at MSU. Explore the application of thermodynamics in engineering, study energy transfer, and understand the principles behind power cycles and refrigeration cycles. Discover prerequisite courses, required textbooks, and the evolution of the curriculum, providing a comprehensive view of this fascinating field.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
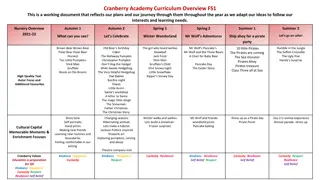
Status of Core Courses: Biothermodynamics Christopher M. Saffron Department of Biosystems and Agricultural Engineering 07/14/14 1 1 1
Introduction to Engineering Thermodynamics Thermodynamics Therme = heat Dynamis = power Science about the conversion and transfer of different forms of energy Energy Viewed as the ability of systems to cause changes A system has energy if it has the capacity to do work To understand energy, you need to understand energy transfer Energy is in the form of heat if an energy transfer is caused by a temperature difference Energy is in the form of work if its transfer is caused by a force acting through a distance 2 2 2
Thermodynamics in Engineering Curriculum Course Description: learn to apply the first and second laws of thermodynamics. Analysis of closed and open systems. Study of power cycles and refrigeration cycles. Study of gas-vapor mixtures and psychrometry. Analysis of reaction equilibria and phase equilibria. Prerequisites at MSU: BE 101. Introduction to Biosystems Engineering MTH 235. Differential Equations (first-order linear equations, exact equations, introduction to PDEs) BS 161. Cells and Molecules (energy metabolism, macromolecules, genetics) Prerequisites at other Universities: Differential Equations, Multivariable Calculus, Introduction to Bio-engineering 3 3 3
Textbooks MSU engel and Boles, Thermodynamics: An Engineering Approach. 8th ed. (required) Haynie, Biological Thermodynamics. 2nd ed. (optional) Other Universities 2 used engel and Boles 1 created a custom text through McGraw Hill titled Thermodynamics for Living Systems ; problems from engel and Boles 1 used Moran et al., Fundamentals of Engineering Thermodynamics, 7th ed. 1 used Borgnakke and Sonntag, Fundamentals of Thermodynamics, 7th ed. 1 used course notes 4 4 4
Context in BE at MSU BS 161 Cells and Molecules MTH 235 - Differential Equations BE 101 - Intro to Biosystems Engineering BE 351 Thermodynamics for Biological Engineering BE 468 Biomass Conversion Engineering BE 478 Food Engineering: Solids BE 477 Food Engineering: Fluids BE 485/487 Senior Capstone Design 6 6 6
History and Evolution of the Course at MSU 1990 s Focus on power cycles (including jet engines) Implies coverage of flow-through devices (open systems) Second law 2000 to 2006 Course title: Environmental Thermodynamics Syllabus includes closed systems, open systems, cycles and psychrometry 2007 to present Course title changed to Thermodynamics for Biological Engineering Engineering process focused with biological engineering applications 7 7 7
Other Engineering Thermodynamics Courses ME 201 Thermodynamics (3 credit hours) Basic concepts of thermodynamics. Property evaluation of ideal gases and compressible substances. Theory and application of the first and second laws of thermodynamics. Entropy and Carnot efficiency. CHE 321 Thermodynamics for Chemical Engineering (4 credit hours) First and second laws. Thermodynamics of flow and energy conversion processes. Properties of single and multi-component systems. Phase equilibria. Chemical equilibria in reacting systems. ENE 481 Environmental Chemistry: Equilibrium Concepts (3 credit hours) Chemistry of natural environmental systems and pollutants. Equilibrium concepts and calculations for acid-base, solubility, complexation, redox and phase partitioning reactions and processes. Applications to ecosystem analysis, pollutant fate and transport, and environmental protection. 8 8 8
Teaching Practices ABET Problem solving identified as weakness Thermo is critical for advancing problem solving skills Hybrid lecture and active learning 31 lectures 36 problems solved in class ~50% of class time involves student problem solving 8 in-class quizzes 11 assigned homework problem sets, due about weekly At least one FE-type problem per homework set 3 in-semester exams and a final exam 9 9 9
Laws of thermodynamics First law in any process, the total energy of the universe remains constant, i.e. energy cannot be created or destroyed Second law the entropy of an isolated system not in equilibrium will tend to increase over time, approaching a maximum value at equilibrium energy has quality as well as quantity, a process occurs in the direction of decreasing energy quality Third law as temperature approaches absolute zero, the entropy of a system approaches a constant Zeroth law if two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other 10 10
Energy Transfer 88% Heat The form of energy that is transferred between two systems, or a system and its surroundings, by virtue of a temperature difference An energy interaction is heat only if it takes place because of a temperature difference Heat is energy in transition, it is recognized only as it crosses the boundary of a system Work Energy transfer associated with a force acting through a distance An energy interaction not caused by a temperature difference between a system and its surroundings Work per time is power Whether energy is transferred as heat or work can depend on how the system boundary is selected, e.g.: Electric oven Electric oven Heating element Heating element 11 11
P critical point Properties of pure substances subcooled liquid P, v, T, U, H, S, G, A, Cp, Cv, etc. Internal energy U Enthalpy -- conveniently defined combination property H = U + PV 88% sat d liquid- vapor region Nuclear energy Chemical energy * v T critical point Latent energy subcooled liquid Sensible energy Atom Atom sat d liquid- vapor region 12 12 v *Adapted from Cengel and Boles 7th ed. Thermodynamics: An Engineering Approach
Closed System Energy Balances Analysis of the human body as a closed system Humans are really open systems Chemical reactions occur in cells Metabolism sum total of all chemical reactions in cells Result of burning foods carbohydrates, proteins, fats The rate of metabolism in the resting state is called the basal metabolic rate for an average adult the basal metabolic rate is 72 W the body dissipates energy to the environment at a rate of 72 W chemical energy from food is converted to thermal energy at a rate of 72 W function of activity, e.g. exercise can increase metabolic rate more than 10x 100% F ds A P = + KE + Q W U PE , , net in net out 13 13
Open System Energy Balances 88% Open systems involve mass flow across the system boundaries Work is required to push the mass into or out of the control volume this work is flow work V P m F CV F Imaginary piston Rate of change in internal, Rate of energy Rate of energy = potential, kinetic, energies etc., input output dE E E sys = 0 = in out dt E E = in out 2 2 v v in out Q Q + + + + = + + + + W m h gz W m h gz in in out out 2 2 14 14
Open System Energy Balances for Engineering Devices Nozzles: Turbines: W Q = = 0 0 Typically involve no work: Typically no potential energy change: Large changes in velocity: Heat transfer is usually negligible: Potential energy is negligible: High velocities, but velocity change is low, therefore KE is small compared to H: pe = 0 pe = 0 ke 0 ke h = = w 0 2 2 2 1 v v = Energy balance for nozzles: h Energy balance for turbines: , s out 2 Throttling devices: No change in potential energy: Outlet velocity is greater than inlet velocity, but kinetic energy change is negligible: Compressors and pumps: pe = 0 Q = = = 0 Heat transfer is usually negligible: Potential energy is negligible: Velocities are typically low: Energy balance: pe ke 0 0 ke = 0 = h w h = 0 The energy balance reduces to: Valves are isenthalpic devices in , s 15 15
Open System Energy BalancesUnsteady State Mass balance: ( )CV Wb = m m m initial m in out final Energy balance: Q We = E E E CV in out ( ) in out = + + + + min m e m e Q W m Q W m mout f f i i in in out out CV = + + h ke pe Wsh = + + e u ke pe 16 16
Entropy and the Second Law 88% Clausius Inequality: Energy has quality as well as quantity, and actual processes occur in the direction of decreasing quality The entropy of an isolated system not in equilibrium will tend to increase over time, approaching a maximum value at equilibrium Heat cannot spontaneously flow from a material at lower temperature to a material at higher temperature It is impossible to convert heat completely into work Q T 0 Q = 0 T rev Q = dS Change in the Total Total Total T rev = + total entropy entropy entropy entropy of system the entering leaving generated Gibbs Equations: = = + Tds du Pdv = + S S S S sys in out gen Tds dh vdP Q dS S = + + CV k m s m s i i o o gen dt T k 17
88% 100% 100% Heat Engines, Carnot Cycles, Reversibility W High-temperature source , net Q out = The Carnot heat engine P th H 1 QH QH Q =1 L th Q 2 H Heat engine Wnet,out Wnet,out T =1 L 4 , th rev T QL H 3 Low-temperature sink QL v 18 18
Reciprocating Piston Power Cycles 25% qin qin qout qout Otto Cycle: Diesel Cycle: air air air air air 3 2 2 air 2-3 2 air air 3 3 4-1 4-1 4 4 1 1 Isentropic compression V=const. Heat rejection Isentropic compression V=const. Heat rejection Isentropic expansion V=const. Heat addition Isentropic expansion P=const. Heat addition qin P 2 3 3 P qin 4 4 qout qout 2 1 1 V V BDC TDC 19
Gas and Vapor Power Cycles Rankine Cycle: Brayton Cycle: 38% 38% qin qi n Heat exchanger Boiler 2 3 2 3 Wpump,in Wnet, out Wturb,out Turbine Compressor Turbine 4 1 4 1 Heat exchanger Condenser qout qout Working fluid = Water Working fluid = Air Power plant tour at 2/3 semester 20
Ideal Vapor Compression Refrigeration Cycle Warm 38% environment at TH > TL QH T 2 Condenser 3 2 QH 3 Expansion valve Wnet,in Win Working fluid = Refrigerant 1 4 Evaporator 4 4 1 QL QL Cold refrigerated space at TL s 21
Differential Property Relations 25% Gibbs Equations: Maxwell Relations: Property Relations: P = du Tds Pdv T v T P = + 2 2 u u C dT T P dv = 2 1 v T T v = + dh Tds vdP 1 1 v s v s v = da sdT Pdv T v v T P = = + 2 2 h h C dT v T dP 2 1 P P s = + dg sdT vdP T T P 1 1 s P P s P = C P v T T v = + 2 2 v s s dT dv T v 2 1 T T T v s v 1 1 v = P T 2 C P 2 T P = v T v T T v 22
Air-water Psychrometrics Human comfort: 75% Human body can be viewed as a heat engine with food as the energy input Heat transfer is proportional to the temperature difference between the body and the environment In cold environments, the body will lose more heat because of a larger T difference In response to cold T, the body reduces blood flow near the surface of the skin In hot environments, improving heat dissipation is necessary During light exercise, about half of the rejected body heat is dissipated through perspiration as latent heat, and half is dissipated through convection or radiation as sensible heat When resting, ~70% of heat is dissipated as sensible heat When vigorously exercising ~60% of heat is dissipated as latent heat Human comfort primarily depends upon the dry-bulb temperature, the relative humidity, and the air motion Most people feel comfortable between 22 and 27 C Relative humidity between 40 and 60% (higher relative humidity impedes heat rejection by evaporation) Air velocities of about 15 m/min (high air velocities displace the moist air immediately surrounding the body, and therefore increase heat rejection) Absolute humidity, Dry-bulb temperature Absolute humidity, Humidifying Cooling Heating humidifying De- 23 Dry-bulb temperature
Chemical Reactions 75% p r = i = j = N N h h h i f, i j f, j R 1 1 Fuel CO2 HOH N2 ( ) = + Enthalpy hf h h Combustion Chamber Air Sensible energy ( ) r = j Q + + + = n W j f, h h h j j j in in 1 hc = Hprod Hreact p ( ) = i Q + + + n W h h h i i f, i i out out 1 24 24 24
Chemical and Phase Equilibrium T vapor Criteria for chemical equilibria at a fixed T and P vapor and liquid G 50% liquid 0 dG 0 dG 63% iy 1 0 = 0 dG Henry s Law: P , i gas = y Violation of the 2nd law , i liquid H Raoult s Law: = y total P y P , liquid , , i gas i i sat 100% reactants 100% products Equilibrium composition 25 25 25
Other topics Non-ideal gas behavior 25% Exergy analysis 25% Energy flow in ecosystems 38% Thermodynamics of cell metabolism 25% Atmospheric thermodynamics and climate change Hydrogen, solid oxide and microbial fuel cells Acid-base chemistry, thermochemistry (esp. combustion) Osmosis Chemical kinetics Fluid mechanics Human systems Mass balances 26 26 26
Survey Slide Thermodynamics: Percent of courses included the topic Non-ideal gas behavior Exergy analysis Energy flow in ecosystems Thermdynamics of cell metabolism Phase equilibrium Chemical equilibrium Chemical reactions Air-water psychrometrics Differential property relations Refrigeration cycle Rankine cycle Brayton cycle Reciprocating piston cycles Reversible systems Entropy balances Carnot cycle Heat engines Open system energy balances Closed system energy balances Properties of pure substances Energy transfer 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 27 27 27
Student Feedback Generally, BE Thermodynamics courses are well received MSU feedback states don t go any faster More biological applications desired by students More biomedical engineering thermodynamics Metabolic engineering Pharmacodynamics Thermodynamics of cell metabolism 28 28 28
In summary, the need for Biological Engineering Thermo Traditional ME and CHE thermodynamics don t cover biological applications Limited to no refrigeration Limited to no psychrometry Focus on industrial chemicals in CHE thermo Non-ideal aqueous solutions is standard in biology Discipline relevant topics to consider Energy capture by plants Microbial metabolism Humans as heat engines Energy flow in ecosystems 29 29 29
Thank you! 30 30 30