Recrystallization for Solid Organic Compound Purification
Recrystallization is a laboratory technique used to purify solids based on their different solubilities. It involves dissolving an impure solid in a suitable solvent, cooling the solution to allow pure crystals to form, filtering to isolate the purified solid, and drying it. Finding a good recrystal
1 views • 11 slides
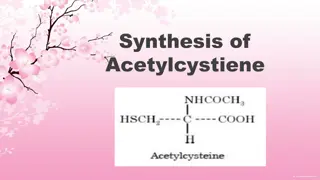
Synthesis of Acetylcysteine: Procedure, Uses, and Precautions
The synthesis of acetylcysteine involves acetylating L-Cysteine in the presence of acetic anhydride and sulfuric acid. Acetylcysteine has various uses, including reducing pulmonary secretions' viscosity, being an antidote for acetaminophen overdose, and chelating heavy metals. The procedure, precaut
0 views • 20 slides
Guide to Recrystallization Techniques for Purifying Solid Organic Compounds
Solid organic compounds obtained from organic reactions are often impure and require purification through recrystallization. This process involves choosing a suitable solvent, testing solubility, and using decolorizing charcoal to remove impurities. Proper solvent selection is crucial for successful
0 views • 13 slides
Preparation of Aspirin: Synthesis and Safety Guidelines
Aspirin, derived from salicylic acid, is synthesized by reacting salicylic acid with acetic anhydride using a catalyst such as sulfuric acid. The product is purified through recrystallization. Safety precautions must be taken due to the hazardous nature of the chemicals involved. The procedure invol
0 views • 12 slides