Understanding Electrochemistry in Engineering Chemistry
Electrochemistry in engineering chemistry explores the interactions between electrical and chemical energy, involving the conversion of energy forms. It discusses electrical conductors, insulators, metallic conductors, good conductors, semiconductors, and electrolytic conductors. The concept of elec
4 views • 56 slides
Understanding Electrophoresis: Principles, Techniques, and Applications
Electrophoresis is a technique dating back to principles of electrochemistry, involving the movement of charged particles in an electric field for separation. Factors affecting electrophoretic mobility include charge, particle size, shape, and applied electrical field. Techniques such as paper, gel,
0 views • 22 slides
Understanding Electrode Reactions in Electrochemistry
Exploring electrode reactions in electrochemistry involves delving into Faraday's law, coulometry, and the importance of sustainable electrode reactions. These concepts help us understand how the quantity of charge passed affects the production or consumption of substances in electrode reactions. As
4 views • 27 slides
Understanding Electrochemistry: Principles and Applications
Electrochemistry is a branch of chemistry focusing on electricity generation from spontaneous chemical reactions and utilizing electrical energy for non-spontaneous transformations. It encompasses important concepts like electrolytes, electrode potential, electrochemical cells, salt bridges, and the
0 views • 20 slides
Advancements in Dye-Sensitized Photoelectrochemical Cells
History traces the development of photoelectrochemical cells since 1972, highlighting the progress, challenges, and state-of-the-art dyes used, with an emphasis on improving performance through innovative solutions like Al2O3 layers. Key issues such as dye degradation and electron-hole recombination
0 views • 15 slides
Understanding Electrochemistry Concepts and Redox Reactions
Explore the fundamentals of electrochemistry, oxidation-reduction reactions, and identification of redox components. Learn about oxidation states, electron transfer, and half-reactions. Dive into examples and visual aids to grasp the concepts easily.
0 views • 42 slides
Understanding Electrochemistry: Redox Reactions, Cells, and Equations
Electrochemistry is a branch of chemistry that involves redox reactions, galvanic cells, standard reduction potentials, balancing redox equations, batteries, corrosion prevention, and electrolysis. Learn about the fundamental principles, examples of redox reactions, and how to balance equations usin
0 views • 61 slides
Understanding Galvanic Cells in Materials Engineering
Exploring galvanic cells in materials engineering, we delve into examples involving zinc, nickel, copper, and platinum electrodes immersed in various electrolytes. By analyzing oxidation and reduction reactions, we determine electrode roles, corrosion tendencies, and cell potentials to understand th
0 views • 28 slides
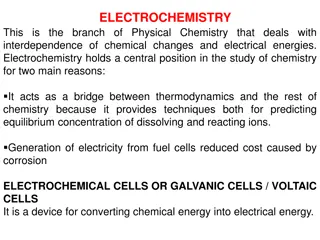
Understanding Electrochemistry: The Interplay of Chemical Changes and Electrical Energies
Electrochemistry, a branch of Physical Chemistry, explores the relationship between chemical reactions and electrical energies. It serves as a vital link between thermodynamics and other areas of chemistry, offering insights into equilibrium concentrations and facilitating the generation of electric
0 views • 24 slides
Welcome to Chemistry 1B Lecture: Introduction and Course Overview
In this Chemistry 1B lecture, Dr. Roy Dixon introduces the course, instructors, teaching goals, and topics to be covered. Students are briefed on class materials, lab expectations, and the importance of problem-solving skills. The session covers equilibrium, thermodynamics, electrochemistry, and mor
0 views • 25 slides
Battery Escape Room: Interactive Science Learning Experience
Engage students in a hands-on learning experience with the Battery Escape Room project funded by the EU's Horizon 2020 programme. In this innovative classroom activity, students are challenged to build batteries using everyday materials, explore electrochemistry concepts, and collaborate to solve pu
0 views • 4 slides
Understanding Electrochemistry: A Comprehensive Overview
Dive into the world of electrochemistry with this detailed review covering topics such as oxidation, reduction, half-reactions, and the significance of oxidation numbers in balancing redox equations. Explore key concepts like OIL RIG (Oxidation Is Loss, Reduction Is Gain) and learn how to apply elec
0 views • 17 slides