Biosafety and IBC - Understanding Research Conduct and Safety Measures
Explore the essential aspects of biosafety and the role of Institutional Biosafety Committees (IBC) in ensuring responsible research conduct. Learn about biohazards, biosecurity, laboratory-acquired infections, routes of exposure, and the structure and functions of the IBC. Enhance your understandin
1 views • 13 slides
Biological Registration Process and Administrative Controls for Safety Focus
Registering work involving biological materials, toxins, and recombinant DNA is essential for safety compliance. Principal Investigators must follow NIH guidelines and register with the Institutional Biosafety Committee. Contact NIH for additional instructions on working with toxins and Select Agent
5 views • 13 slides
Ensuring Biosafety in Laboratory Settings: Barriers and Practices
Biosafety in laboratories involves implementing various barriers and practices to prevent the release of biological agents and protect lab workers. These include primary and secondary barriers, personal protective equipment (PPE), containment principles, and adherence to biosafety practices. Safety
1 views • 41 slides
Overview of Biosafety and Biosecurity in Global Health Security
The paper delivered at the 2022 Scientific Workshop highlights the importance of biosafety and biosecurity in the face of emerging infectious diseases. It defines key terms, emphasizes the need for containment procedures to manage biohazards, and addresses the critical pillars of biosafety and biose
0 views • 45 slides
Understanding Biosafety and Biosecurity Principles
Biosafety and Biosecurity are essential concepts in safeguarding against biological hazards. This article explores the definitions of hazard, threat, and risk, emphasizing the importance of managing risks associated with biological materials through biosafety and biosecurity measures. Learn about th
0 views • 26 slides
Understanding Biohazards: Levels, Safety Precautions, and Risk Groups
Biological hazards or biohazards pose risks to human health through organisms like bacteria, viruses, parasites, and fungi. Biosafety and biosecurity measures are crucial to prevent exposure, with different biosafety levels and risk group classifications guiding safety protocols in laboratory settin
2 views • 12 slides
Laboratory-Acquired Infections: Routes of Exposure and Prevention Measures
The content discusses laboratory-acquired infections from 1930 to 2015 and highlights exposure routes such as facial mucous membranes, percutaneous, ingestion, inhalation, and non-traditional routes like eye and nasal cavity. It emphasizes the importance of biosafety practices, identifies GI pathoge
0 views • 21 slides
Understanding Biosafety and Containment Measures in Laboratories
This content provides comprehensive information on biosafety, containment, biohazards, and the importance of preventing laboratory acquired infections. It covers the principles, practices, and procedures designed to safeguard against biological agents and toxins. The concept of biosecurity, containm
3 views • 17 slides
Safe Handling of Hazardous Drugs - Ventilation Tool Cleaning Procedures
This presentation outlines the proper procedures for cleaning, disinfecting, and organizing ventilation tools, emphasizing the importance of trained operators following written protocols. It covers waste handling, documentation, and the use of personal protective equipment (PPE) for occupational saf
0 views • 24 slides
Cutting-Edge Research Equipment and Studies at The Aga Khan University, Pakistan
The research facilities at The Aga Khan University in Pakistan boast state-of-the-art equipment for groundbreaking studies in areas like enteric fever prevention, rotavirus vaccine impact assessment, and dental stem cells. Researchers utilize specialized tools such as refrigerated centrifuges, biosa
0 views • 29 slides
Closing the Gap Between Biorisk Policy and Implementation in Africa
This presentation by Dr. Louise Bezuidenhout from the University of the Witwatersrand, South Africa, discusses the challenges and current developments in biosafety and biosecurity regulation in Africa. It outlines the research, funding, and policy landscape, focusing on the gap between policy formul
0 views • 14 slides
GMO Regulations in Sri Lanka: An Overview of Biosafety and Prohibitions
Sri Lanka's biosafety regulations of 2019 exempt certain cases involving genetically modified (GM) ingredients in processed foods. The regulations outline prohibitions on the import, sale, and distribution of GM organisms as food without approval. Additionally, the regulations address risks in proce
0 views • 12 slides
Collection and Transport of Specimens in Diagnostic Microbiology
Successful laboratory investigations depend on the proper collection and transport of specimens. This involves selecting adequate samples that represent the diseased area, avoiding contamination, obtaining specimens before administering antimicrobials, ensuring biosafety, proper documentation, and t
0 views • 20 slides
Addressing Land Degradation Challenges in Agriculture for Sustainable Development
World agriculture has evolved over the decades, facing challenges like environmental degradation and land misuse. Awareness-raising activities on biosafety and biodiversity are crucial for informed decision-making in agriculture. Nigeria, with its diverse ecological zones, must sustainably manage na
0 views • 13 slides
Biosafety Training Requirements for Research Group Members
Principal Investigators with a Biological Use Authorization at UC Berkeley must provide lab-specific biosafety training to research group members. This training template covers CLEB administrative requirements, risk assessment, good work practices, emergency procedures, and more. Compliance ensures
0 views • 17 slides
Guide to UCSB Biological Safety Program
This guide provides an overview of UCSB's Biological Safety Program, covering important aspects such as lab safety fundamentals, biological use authorization, biosafety officer's role, and the Institutional Biosafety Committee. It outlines key steps like hazard assessment, training, waste management
0 views • 24 slides
Recombinant DNA Training Overview for Research Group Members
This training template is designed to assist Principal Investigators in providing lab-specific biosafety training to research group members working with recombinant DNA. It covers essential topics such as NIH guidelines, administrative requirements, risk assessment, emergency procedures, and more. C
0 views • 22 slides
SHERM's Impact and Response to COVID-19 at UTHealth
COVID-19 pandemic significantly influenced departmental operations at UTHealth, with services either increasing, maintaining, or deferring. SHERM staff, essential personnel working on-campus, adapted to high workloads and participated in furlough programs. Biosafety measures, travel restrictions, re
0 views • 46 slides
Understanding Risk Assessment for Biosafety in the Caribbean Sub-Region
Risk assessment is a structured approach to evaluating the potential harm from activities involving genetically modified organisms (GMOs) in order to protect health and the environment. It involves identifying, characterizing, and evaluating risks to determine the level of concern and the need for m
0 views • 16 slides
Understanding NIH Guidelines and Virginia Tech's Institutional Biosafety Committee
The NIH Guidelines and Virginia Tech's Institutional Biosafety Committee play crucial roles in ensuring safety and compliance in research involving recombinant and synthetic nucleic acids. The guidelines outline biosafety practices and procedures, while the committee oversees the establishment of po
0 views • 24 slides
Understanding COMS and Recombinant DNA Regulations
The Committee on Microbiological Safety (COMS) was established in 1978 to address public concerns regarding safety, environment, and ethics of research involving hazardous biological agents. COMS oversees activities related to recombinant DNA and biological agents at Harvard, supporting all schools
0 views • 6 slides
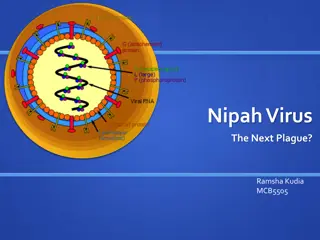
Understanding Nipah Virus: A Comprehensive Overview
Nipah virus, belonging to the Paramyxoviridae family, is a zoonotic virus with high pathogenicity and mortality rates. It falls under the Henipavirus genus, known for infecting a wide range of animal species. Its virion structure consists of non-segmented, negative-sense RNA, and the viral genome co
0 views • 22 slides