Clinical Trial Supplies Market to be Worth $5.59 Billion by 2031
Clinical Trial Supplies Market by Phase (I\u2013IV), Service (Manufacturing, Packaging, Logistics, Documentation), Type (Biologics, Small Molecules, Medical Devices), Therapeutic Area (Oncology, Cardiology, CNS, Immunology, Respiratory), End User, and Geography - Global Forecast to 2031
3 views • 4 slides
Guidelines for Expanded Access to Investigational Drugs and Devices
Providing guidance on types of Expanded Access for drugs, biologics, and devices, this content explains the concept of Expanded Access and the criteria that must be met. It covers different types of Expanded Access for drugs and devices, emphasizing the importance of patient safety and benefit. The
0 views • 10 slides
Advancing Healthcare: Potential of Advanced Amniotic Tissue by Life Biologics
Life Biologics is at the forefront of using amniotic tissue to develop premium human amniotic tissue grafts for advanced wound care. Our innovative products offer patients a comprehensive range of treatment options. Life Biologics' advanced skin substitutes provide a revolutionary solution for both
0 views • 5 slides
MTF Biologics: Advancing Healthcare Through Tissue Donation
MTF Biologics is a global nonprofit organization dedicated to saving and healing lives through tissue and organ transplantation. They uphold high standards, provide exceptional services, and support healthcare providers, donors, patients, clinicians, and researchers. By transforming, collaborating,
0 views • 19 slides
Understanding Investigational Products in Clinical Trials
Investigational products play a crucial role in clinical trials, encompassing drugs, devices, biologics, and more. The FDA defines investigational new drugs as substances seeking approval, even if previously in use, with potential changes. Similarly, investigational devices are those under investiga
0 views • 43 slides
Biosimilars in Modern Healthcare: Trends and Insights
Exploring the landscape of biosimilars, this content delves into the regulatory aspects, interchangeability, formulary management, and future implications of these biologic alternatives. With a focus on the dominance of biologics in the market, comparisons with small molecule drugs, and the complexi
0 views • 40 slides
Wound Biologics Market Key Players Focus on Innovations 2023-2030
The global wound biologics market was valued at $1,851.4 million in 2022 and is anticipated to grow to $3,321.5 million by 2030, with a CAGR of 7.71% during the forecast period 2023-2030.\nRead Report Overview: \/\/bisresearch.com\/industry-report\/g
1 views • 3 slides
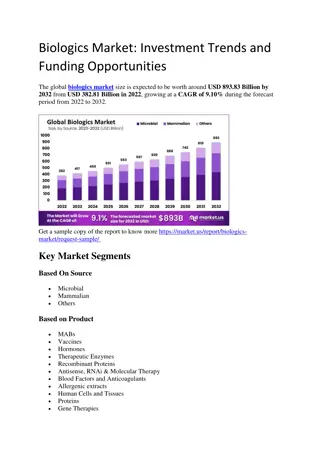
Biologics Market
The global\u00a0biologics market\u00a0size is expected to be worth around\u00a0USD 893.83 Billion by 2032\u00a0from\u00a0USD 382.81 Billion in 2022, growing at a\u00a0CAGR of 9.10%\u00a0during the forecast period from 2022 to 2032.
0 views • 4 slides
Understanding U.S. Physician Payment Sunshine Act
The U.S. Physician Payment Sunshine Act mandates transparency in reporting payments and transfers of value from pharmaceutical, biologics, and medical device manufacturers to U.S. physicians and teaching hospitals. Reporting requirements include details like recipient information, payment amounts, a
0 views • 9 slides
Understanding the Structure and Functions of FDA in the USA and Canada
The Food and Drug Administration (FDA) in the USA is a critical agency within the Department of Health and Human Services responsible for regulating the safety of various products such as foods, drugs, medical devices, and cosmetics. The FDA has distinct organizational units like the Office of the C
0 views • 49 slides
Understanding FDA Regulations in Clinical Research
FDA regulations in clinical research cover the protection of human subjects, IND/IDE requirements, risk determinations, application processes, and what falls under FDA regulation. It distinguishes between FDA-regulated activities and those not regulated, such as medical record reviews. The scope inc
0 views • 31 slides
Yearly Biologic Product Report (YBPR) Template Overview
The Yearly Biologic Product Report (YBPR) is a crucial document submitted annually by manufacturers of Schedule D biologic drugs, providing production information on drug substance and product lots. This report serves as a formal tool for post-market quality information, ensuring consistency, safety
0 views • 37 slides
Understanding Biosimilars: Tools for Analytical Similarity
Explore the world of biosimilars, the complexities of comparing generic and brand drugs, FDA regulations, and the first FDA-approved biosimilar. Learn about biological products, the Biologics Price Competition and Innovation Act, and the challenges in defining potency, purity, and stability in biolo
0 views • 26 slides
Understanding Investigational Products in Clinical Trials
Investigational products play a crucial role in clinical trials, encompassing drugs, devices, and biologics that undergo testing to ensure compliance with protocols and participant safety. This comprehensive guide covers the definitions, regulations, and accountability of investigational products in
0 views • 42 slides
Evolution of Regulation in the Pharmaceutical Industry
The pharmaceutical industry plays a crucial role in discovering, developing, and marketing drugs for patient use. Historical incidents of unsafe drugs led to the implementation of regulations such as the Biologics Control Act of 1902 and the Pure Food and Drugs Act of 1906. These laws aimed to ensur
0 views • 11 slides