Understanding Ocean Acidification: Impact on Coral Reefs
Ocean acidification is the process where oceans become more acidic due to excessive carbon dioxide absorption. Human activities like deforestation and vehicle emissions contribute to this phenomenon. Coral reefs are crucial as many ocean species rely on them for survival. The pH scale measures acidi
0 views • 18 slides
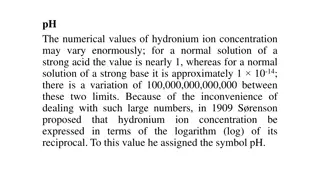
Understanding pH Scale and Hydronium Ion Concentration
The pH scale is a logarithmic measurement that indicates the acidity or alkalinity of a solution based on the concentration of hydronium ions. pH values range from 0 to 14, with 7 being neutral. Lower pH values indicate acidity, while higher values indicate alkalinity. This scale provides a convenie
0 views • 104 slides
Understanding Citrus Nutrient Availability in Relation to Soil pH and Irrigation Water Acidification
This research by Dr. Kelly T. Morgan from the University of Florida explores the impact of soil pH, irrigation water acidification, and nutrient recommendations on citrus trees affected by HLB disease. The study examines the availability of key nutrients such as Mn and Zn, the effects of different s
0 views • 23 slides
Understanding Acids and Bases: pH Scale Explained
Exploring the pH scale, this content delves into the fundamentals of acidity and alkalinity, covering what pH stands for, the inventor of the pH scale, reactions when acids and bases combine, and where the weakest acids and bases are found on the pH scale.
0 views • 7 slides
Milk Quality Assurance: Detection of Neutralizers, Adulterants, and Preservatives
This study focuses on techniques for estimating neutralizers, adulterants, and preservatives in milk and milk products, including tests for Rosalic Acid, alkalinity of ash, sugar, starch, and glucose. Each test involves specific procedures using apparatus and reagents to detect the presence of certa
0 views • 17 slides
Understanding Acids, Alkalis, Indicators, and the pH Scale
Solutions can be acidic, alkaline, or neutral depending on whether an acid or alkali is dissolved in water. Indicators like litmus and universal indicator help determine acidity or alkalinity. The pH scale measures this, with 7 being neutral. Neutralization reactions occur when an acid and a base re
0 views • 5 slides
Estimation of Alkalinity in Water Sample Practical for B.Sc. III Year
Alkalinity in water samples can be estimated by titrating with HCl and using indicators like Phenolpthalein and Methyl orange. The presence of OH-, CO3-, and HCO3- contributes to alkalinity, giving pink or yellow colors with different indicators. The process involves adding indicators, titrating wit
0 views • 7 slides
Piseco Lake Environmental Data Analysis
This report highlights the environmental characteristics of Piseco Lake, including its physical and chemical data. The lake's water quality, transparency, temperature, dissolved oxygen levels, pH, alkalinity, phosphorous, nitrates, and chlorophyll levels are analyzed. The data shows trends indicatin
0 views • 21 slides
Understanding Water Composition for Brewing Beer
The minerals and salts in brewing water significantly impact pH levels, particularly in all-grain brewing, affecting flavor contribution. Maintaining ideal mash pH and alkalinity is crucial for enzymatic activity during the brewing process. Alkalinity helps neutralize acids and influences various re
0 views • 16 slides
Understanding Water Quality Parameters and Characteristics
Water quality encompasses physical, chemical, and biological characteristics that determine its suitability for various uses. Physical parameters like turbidity, taste, odor, color, and temperature affect sensory perception. Chemical parameters such as pH, acidity, alkalinity, and hardness relate to
0 views • 26 slides