Understanding Real Gas Behavior in Chemistry
Real gases deviate from ideal gas behavior due to factors like finite volume of gas particles and intermolecular attractions. At high pressures and low temperatures, real gases don't follow ideal gas laws, impacting their molar volume and pressure calculations. This deviation is crucial in analyzing gas behavior accurately.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Thursday, October 8th Happy Friday Row 3 on your pink sheet: 10.6-10.7 review questions Row 4 on your pink sheet: 10.6 practice problems Take out your 10.9 notes question #s 24 and 81(a-c only) are your warmup
Collecting Gases Gases are often collected by having them displace water from a container. The problem is that because water evaporates, there is also water vapor in the collected gas. The partial pressure of the water vapor, called the vapor pressure, depends only on the temperature. You can use a table to find out the partial pressure of the water vapor in the gas you collect. If you collect a gas sample with a total pressure of 758.2 mmHg* at 25 C, the partial pressure of the water vapor will be 23.78 mmHg, so the partial pressure of the dry gas will be 734.4 mmHg. See Table 5.4*
10.9: Ideal Gas Law Deviations (let s get real)
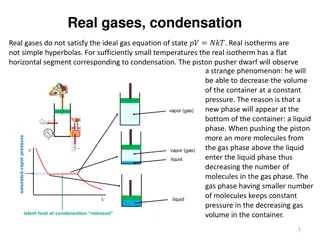
Real Gases Real gases often do not behave like ideal gases at high pressure or low temperature. Ideal gas laws assume 1. no attractions between gas molecules. 2. gas molecules do not take up space. Based on the kinetic-molecular theory At low temperatures and high pressures these assumptions are not valid.
The Effect of the Finite Volume of Gas Particles At low pressures, the molar volume of argon is nearly identical to that of an ideal gas. But as the pressure increases, the molar volume of argon becomes greater than that of an ideal gas. At the higher pressures, the argon atoms themselves occupy a significant portion of the gas volume, making the actual volume greater than that predicted by the ideal gas law. At high pressure, the ideal gas law underestimates volume.
Real Gas Behavior Because real molecules take up space, the molar volume of a real gas is larger than predicted by the ideal gas law at high pressures.
The Effect of Intermolecular Attractions At high temperature, the pressure of the gases is nearly identical to that of an ideal gas. But at lower temperatures, the pressure of gases is less than that of an ideal gas. At the lower temperatures, the gas atoms spend more time interacting with each other and less time colliding with the walls, making the actual pressure less than that predicted by the ideal gas law. At low temperatures, the ideal gas law overestimates pressure
Real Gases A plot of PV/RT versus P for 1 mole of a gas shows the difference between real and ideal gases. It reveals a curve that shows the PV/RT ratio for a real gas is generally lower than ideal for low pressures meaning that the most important factor is the intermolecular attractions. It reveals a curve that shows the PV/RT ratio for a real gas is generally higher than ideal for high pressures meaning that the most important factor is the molecular volume.
Homework Reaction stoichiometry involving gases 71, 73, 75, 77, 79 (old page) Gas challenge questions 130-138 (new page) Gas test on TUESDAY Wednesday: lab Thursday/Friday: start chapter 15!