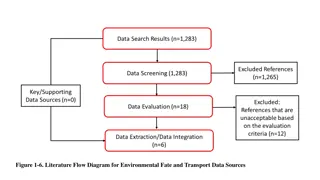
Understanding Cyanide: Sources, Exposure, and Risks
Cyanide is a toxic compound found in various forms such as gases, solids, and liquids that can be harmful to humans and animals. Sources of cyanide exposure include natural processes, industrial activities, plant sources, and combustion products like cigarette smoke. The extent of poisoning depends on the amount and route of exposure. Learn about cyanide detection, risks of exposure through breathing, ingestion, and more, as well as safety regulations to limit exposure to cyanide gas.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Cyanide Lab Cyanide Detection in Samples by Dr. Jessica Smith-Rohrberg is licensed under CC BY 4.0
Cyanide A cyanide is any chemical compound that contains the cyano group (C N), which consists of a carbon atom triple-bonded to a nitrogen atom. Of the many kinds of cyanide compounds, some are gases; others are solids or liquids. Those that can release the cyanide ion CN are highly toxic to animals. Cyanide can be a colorless gas, such as hydrogen cyanide (HCN) or cyanogen chloride (CNCl), or a powdered form such as sodium cyanide (NaCN) or potassium cyanide (KCN). Cyanide sometimes is described as having a bitter almond smell, but it does not always give off an odor around 20-40% of people lack the gene to detect it.
You may be exposed to cyanide by breathing air, drinking water, eating food, or touching soil that contains cyanide. Cyanide enters water, soil, or air as a result of both natural processes and industrial activities. In air, cyanide is present mainly as gaseous hydrogen cyanide It is used in industries such as mining. Smoking cigarettes is probably one of the major sources of cyanide exposure for people who do not work in cyanide-related industries. According to the CDC, cyanide is contained in cigarette smoke and the combustion products of synthetic materials such as plastics (combustion products are substances given off when things burn).
Cyanide is released from natural substances in certain plants and the pits of fruits such as apricots and peaches. These are often found in the seeds to protect the seed from herbivores due to their bitter taste. It is also found in leafs such as spinach. Cyanide is contained in cigarette smoke and the combustion products of synthetic materials such as plastics. Combustion products are substances given off when things burn. Cyanides are produced by certain bacteria, fungi, and algae. Cassava roots (the base from which tapioca is made), also contain cyanide products. However, baking, drying, or soaking these roots renders the cyanide inactive and non-toxic. In manufacturing, cyanide is used to make paper, textiles, and plastics. It is present in chemicals used to develop photographs Cyanide salts are used in metallurgy for electroplating, metal cleaning, and removing gold from its ore. Cyanide gas is often used to exterminate insects and rats in ships and buildings.
Cyanide Gas The extent of poisoning depends on the amount of cyanide a person is exposed to, the route of exposure, and the length of time that a person is exposed. Breathing cyanide gas causes is the most dangerous, but ingesting (swallowing) cyanide can be toxic as well Cyanide gas is most dangerous in enclosed places where the gas is trapped Cyanide gas evaporates and disperses quickly in open spaces, making it less harmful outdoors Cyanide gas is less dense than air, so it will rise. OSHA sets levels of hydrogen cyanide that are allowable in workplace air at10 ppm or 11 milligrams of cyanide per cubic meter of air (mg/m3) averaged over an 8-hour workday and 40-hour workweek. Empty canisters used to contain cyanide gas
Toxicity A concentration of only 300 parts of Hydrogen Cyanide (HCN) per million parts of air can kill a person within a few minutes. This was first used in WWI as a chemical weapon. Zyklon B, a cyanide-based pesticide, was used in the gas chambers in WWII. One teaspoon of a 2% cyanide solution can kill a person. 50-100mg of cyanide is lethal. Cyanide prevents the cells of the body from using oxygen. Because the cells are not getting enough oxygen, heart rate and respiration increase. Cyanide is more harmful to the heart and brain than to other organs because the heart and brain use a lot of oxygen as an energy source without oxygen, the cells in these organs will die. Size matters smaller people are sensitive to smaller amounts.
Symptoms of Cyanide Poisoning People exposed to a small amount of cyanide by breathing it, absorbing it through their skin, or eating foods that contain it may have some or all of the following symptoms within minutes: Rapid breathing Restlessness Dizziness Weakness Headache Nausea and vomiting Rapid heart rate Exposure to a large amount of cyanide by any route may cause these other health effects as well: Seizures Low blood pressure Slow heart rate Loss of consciousness Lung injury Respiratory failure leading to death Showing these signs and symptoms does not necessarily mean that a person has been exposed to cyanide.
In the Body It binds to iron within the hemoglobin of red blood cells this molecule transports oxygen from your lungs to your cells and at the cells, the cyanide binds to an enzyme (cytochrome oxidase), preventing the cells from using oxygen Most cyanide that enters the body is changed to the less toxic form, thiocyanate. Some cyanide reacts to form vitamin B12. The primary route of elimination is through excretion of thiocyanate in the urine. Some free hydrogen cyanide is also excreted unchanged in the breath, saliva, sweat, and urine. Most cyanide and its products leave the body within 24 hours after exposure. After being ingested or inhaled, hydrogen cyanide is rapidly absorbed through the gastrointestinal tract and lungs into the bloodstream. A water-soluble compound, it does not accumulate in the body.
Cyanide in Medicine A cyanide compound, sodium nitroprusside, is occasionally used in emergency medical situations to produce a rapid decrease in blood pressure in humans; it is also used as a vasodilator in cardiovascular research. Japanese physicians in World War I used a copper cyanide compound to treat tuberculosis and leprosy. Note: sodium nitrite is used today as a treatment for cyanide poisoning. Person with leprosy today we treat this bacterial infection with antibiotics.
Cyanide in Liquid 5.0 to 7.2 micrograms per liter: Impacts swimming performance and reproduction in many species of fish 20 to 76 micrograms per liter: death of many species Over 200 micrograms per liter: rapidly toxic to most species of fish 18 to 43 micrograms per liter: Invertebrates: adverse nonlethal effects 30 to 100 micrograms per liter lethal effects to invertebrates The EPA sets regulations for the amount of cyanide allowed in drinking water. The highest amount allowed is 200 micrograms of cyanide per liter of water ( g/L or 0.2 ppm). Cyanide in waterways can evaporate into hydrogen cyanide gas Dead fish from the Tisza, Romania, cyanide disaster
Cigarettes and Cyanide The mainstream smoke of one cigarette may contain between 10 and 500 g hydrogen cyanide. Secondhand smoke may contain between 0.006 and 134 g HCN. The EPA has determined that inhaling quantities over 3 g of HCN may cause non- cancerous side effects. Other data suggest that breathing in 1,000,000 g (1mL) of HCN in 1 minute is toxic. Look at all this different data. Why are there so many discrepancies in amounts? (for example, the difference between 10 and 500)
Apple seeds The body is able to detoxify small amounts of cyanide you would need to eat 100 grams (about a cup, approximately 10 grams of HCN) of apple seeds in one sitting to get sick. Also, the seeds of fruits are very tough a strategy that fruit-bearing plants use to reproduce is to be eaten and excreted by animals. They need a durable outer coating to remain intact while going through the digestive system of animals. However, chewing the seeds breaks down this protective layer. Swallowing apple seeds, even large amounts, therefore isn t as dangerous.
You will be dissolving your contaminated soil samples the contaminants that we will be testing for (lead and cyanide) should separate from the organic matter and we will be able to test for their presence in water. Prepare samples by measuring out: 1. 2g of soil 2. Place into a 150mL beaker. 3. Fill with 80mL of DI water. 4. Label with tape: Sample 1 = Contaminated Soil; Sample 2: whatever was brought from home. 5. If your group has more than one sample from home, work it out so that they all get done. Follow the same procedures.
Cyanide Testing Methods 1. Label a sampling tube N for Negative Control 2. Label a sampling tube 1 for Sample 1/Contaminated Soil 3. Label a sampling tube 2 for your sample from home. 4. Add 5mL of distilled water to the Negative Control test tube. 5. Add 5mL of water from Sample 1 to Sample 1 test tube 6. Add 5mL of water from Sample 2 to the Sample 2 test tube. 7. To each test tube, add: 1. 1 drop of sodium hydroxide (NaOH) 2. 5 drops of ferrous sulfate 3. 5 drops of hydrochloric acid (HCl). 8. Put caps on and shake. 9. If solution turns red, it is positive for cyanide. If it stays the same color, it is negative for cyanide.