Essentials of Site Audits for Global HIV & TB Programs
Understanding the purpose, stages, and requirements of site audits is vital for ensuring accuracy and reliability in test results for Global HIV & TB programs. From identifying improvement areas to implementing corrective actions, this content provides a comprehensive guide for conducting effective site audits.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Introduction to Site Audits Introduction to Site Audits Understanding the requirements for a site audit Division of Global HIV & TB
Content Overview Purpose of an audit Audit stages and requirements Method to audits Audit summation report and dissemination of findings Ethical considerations Training Approach Division of Global HIV & TB
Basic Terms Audit Auditor Person with the competence to conduct an audit Systematic check or audit, especially of the efficiency or effectiveness of an organization or a process, typically carried out by an independent auditor 3
Purpose of an Audit Identify areas where improvement is needed Develop and implement a work plan to address gaps Implement quality assurance elements Monitor quality progress Maintain continuous quality improvement The goal is to ensure the client receives accurate and reliable test results! Division of Global HIV & TB 4
Audit Stages and Requirements Work with MOH and IPs to identify and prioritize sites Notify sites & agree on audit dates Plan for transportation logistics Sensitization meetings with regions and district level teams to convey importance of the SPI-RRT checklist Familiarize yourself with the checklist Review national guidelines and recency study protocol Print additional copies of the checklist for sites Gather any other resources needed (including site visit authorization letter) Pre - Audit Introduce audit team to regional or district health management, including HIV and recency program coordinator when applicable Introduce audit team to facility management Discuss purpose of the audit and the testing points to be visited Describe the audit process including areas to observe, documents to review and provide an estimated time of completion Conduct audit using the SPI-RRT checklist Audit Debrief with the testing point and/or facility management on findings and recommendations Agree on findings, recommendations and timeline for implementing corrective action Identify a point of contact for follow up actions File data at central facility or where applicable based on study protocol Provide feedback within a reasonable timeframe (per national guidelines and study protocol) Post - Audit Division of Global HIV & TB 5
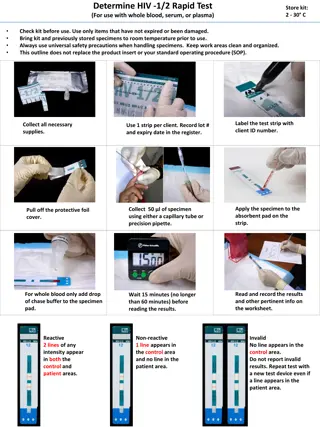
Methods Used to Evaluate Test Site Operations Review the RT site records and documents Observe the RT site operations Ask open- ended questions Follow client specimen or test result through the testing process Division of Global HIV & TB 6
Key Information in Audit Report Part D. Auditor s Summation Report for SPI-RRT Audit Total Facility Name: No. of Tester(s): Section 1 2 3 4 5 6 7 8 Site information Date of audits Overall performance Best practices and deficiencies Corrective actions Timelines Point of contact for follow up Auditor(s) contact Score Received a = Site Type: Audit Start Time (hh:mm) : Expected Score 10 5 10 13 9 9 8 11 b = Site code (if applicable): Audit End Time (hh:mm): % Score = (a/b) x 100 = (_____________/_________) x 100 = __________% Staff Audited Name: Duration of Audit: Performance Level: 0 1 2 3 4 (<40%) (40-59%) (60-79%) (89-90%) (>90%) Correction Actions Recommendations Auditor s Section No. Deficiency/Issue observed Timeline / Person responsible Comments Immediate Follow up Actions Division of Global HIV & TB 7
Dissemination of Audit Findings Information is provided to: NRL and Program coordinators Regional/District program coordinator Facility managers Quality Assurance officer Head of testing sites HIV rapid and recency testing sites personnel Implementing partners Division of Global HIV & TB 8
Key Messages on Audit Process Collaboration CDC country teams, MOH and implementing partners Role of key stakeholders in the process Building on existing site audit implementation Knowledge National guidelines and study protocol Procedures on safety, testing, documentation, etc. Approach Making the site staff comfortable at the start of your visit so they are more open and receptive Emphasize the purpose of the audit is not for disciplinary action but for continuous quality improvement Be respectful the tone you use when providing feedback is important Division of Global HIV & TB
Key Messages on Audit Process contd Methods Observe the test site operations Review records Ask open ended questions Follow client specimen or test result through the testing process Feedback during the audit vs. the debrief mentorship side by side with auditing Opportunity for teaching moments Sandwich Technique Immediate corrective action vs Follow-up Gaps that directly impact testing and safety Division of Global HIV & TB
Implementing Corrective Action Opportunity for mentorship and coaching to address gaps Immediate corrective action vs. Follow-up Gaps that impact providing the right result to the client or the safety of the tester, client or environment On site refresher training Safety Practices Proper blood collection Test procedures Documentation HTS register Data capture form or tablet ODK Feedback = Opportunity for teaching moments Knowledge is critical National guidelines Procedures on safety, testing, documentation, etc. Division of Global HIV & TB 11
Take Home Messages Site audit Provides a solid foundation for ensuring the quality of HIV testing Specifies detailed requirements to conduct an audit Serves as a tool to evaluate a Rapid Testing site against the requirement for quality improvement Acts as guidelines for development of policies and procedures Division of Global HIV & TB 12
Review What is the purpose of an audit? What are the requirements before, during and after an audit? What information should be included in the audit summation report? Division of Global HIV & TB 13